Active tuberculosis marker, kit, detection method and model construction method
A technology of tuberculosis and markers, applied in the field of biomedicine, can solve the problems of low sensitivity and poor specificity, and achieve the effect of high sensitivity, good specificity and good clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Isolation of plasma samples from whole blood
[0055] The fasting peripheral blood of patients with active pulmonary tuberculosis was collected in the morning with EDTA anticoagulant tubes, centrifuged at 3000 rcf for 15 min, and the plasma was separated into a new 1.5 mL centrifuge tube within 6 hours. Plasma samples were stored in a -80°C freezer.
Embodiment 2
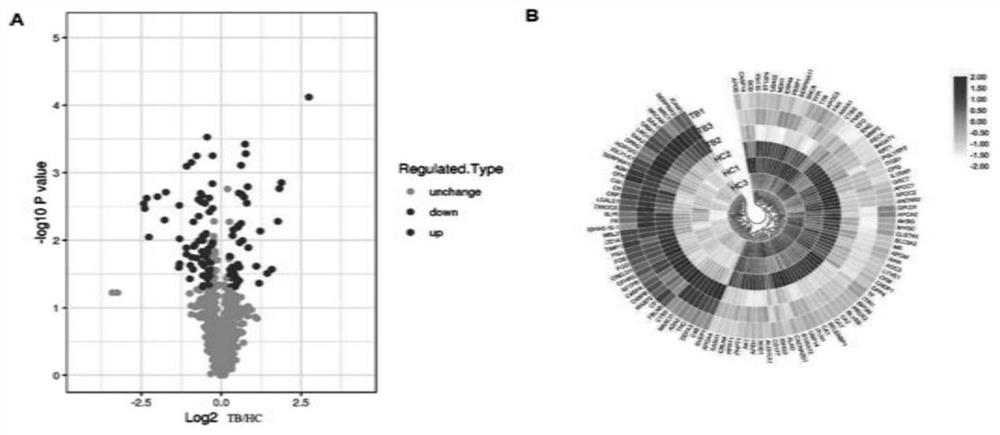
[0057] Quantitative proteomic analysis of plasma samples
[0058] High-abundance plasma proteins were removed using Pierce Top 12 Abundant Protein Depletion SpinColumns Kit (Thermo, USA) according to the kit instructions. The protein solution was reduced with 5mM dithiothreitol, digested at 56°C for 30min, alkylated with 11mM iodoacetamide, and digested at room temperature for 15min in the dark. Then add 100mM TEAB to dilute the protein sample to a urea concentration of less than 2M. Finally, trypsin was added at a trypsin-protein mass ratio of 1:50 for the first digestion, and a trypsin-protein mass ratio of 1:100 was added for the second digestion for 4 h. After trypsinization, the samples were desalted using a Strata X C18 solid-phase extraction column (Phenomenex) and dried in vacuo. Sample peptides were reconstituted in 0.5M TEAB and labeled with TMT kit. Agilent 300Extend C18 column was used for high performance liquid chromatography (HPLC) separation. The processed ...
Embodiment 3
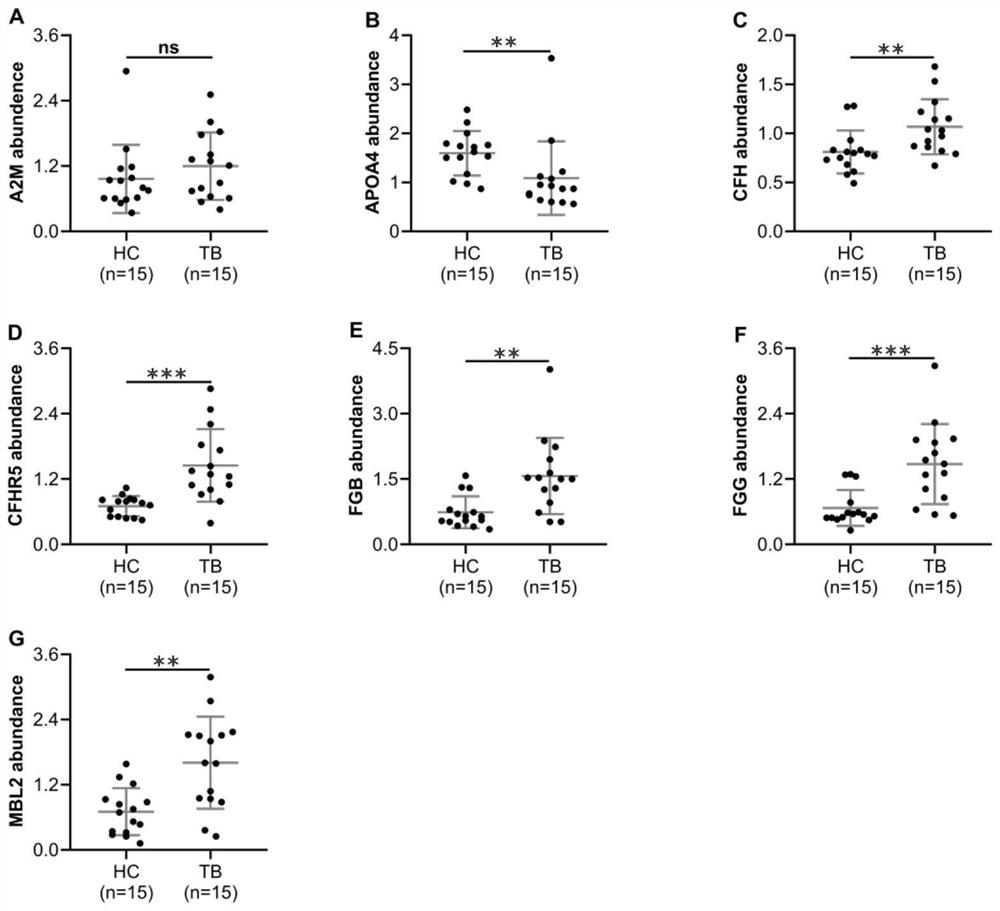
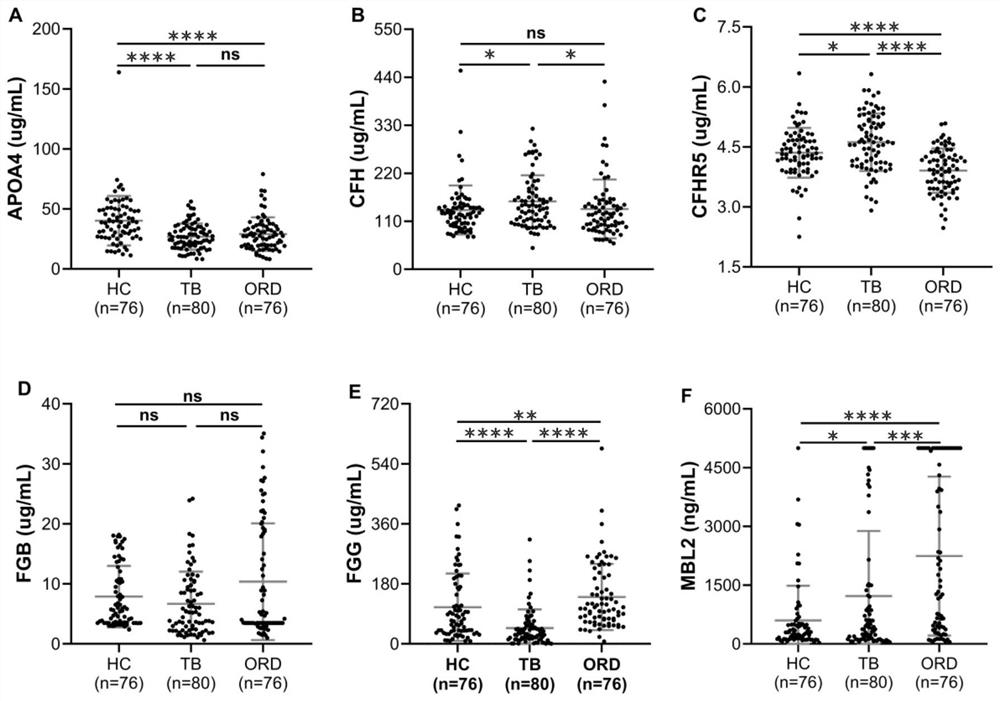
[0060] Determination of Protein Expression Level in Plasma Samples by ELISA
[0061] Sample collection: 80 cases of active tuberculosis patients, 76 cases of healthy controls, and 76 cases of non-tuberculosis pulmonary respiratory diseases.
[0062] Human APOA4 and MBL2 ELISA kits (Elabscience, WuHan, CN) and human CFH, CFHR5, FGB, FGG ELISA kits (Cloud-Clone Corp, WuHan, CN) were used to detect the expression levels of plasma protein markers according to standard operations. The t test or one-way ANOVA in GraphPad Prism 8 software was used for statistical analysis (when P<0.05, there was a significant difference, and when P<0.01, there was an extremely significant difference). Differentially expressed plasma protein data were expressed as mean±SEM, and a scatter plot containing error bars was drawn.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com