Compound and application thereof to detection of mycobacterium tuberculosis
A technology of compounds and derivatives, applied in the field of detection of Mycobacterium tuberculosis, can solve problems that need to be developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
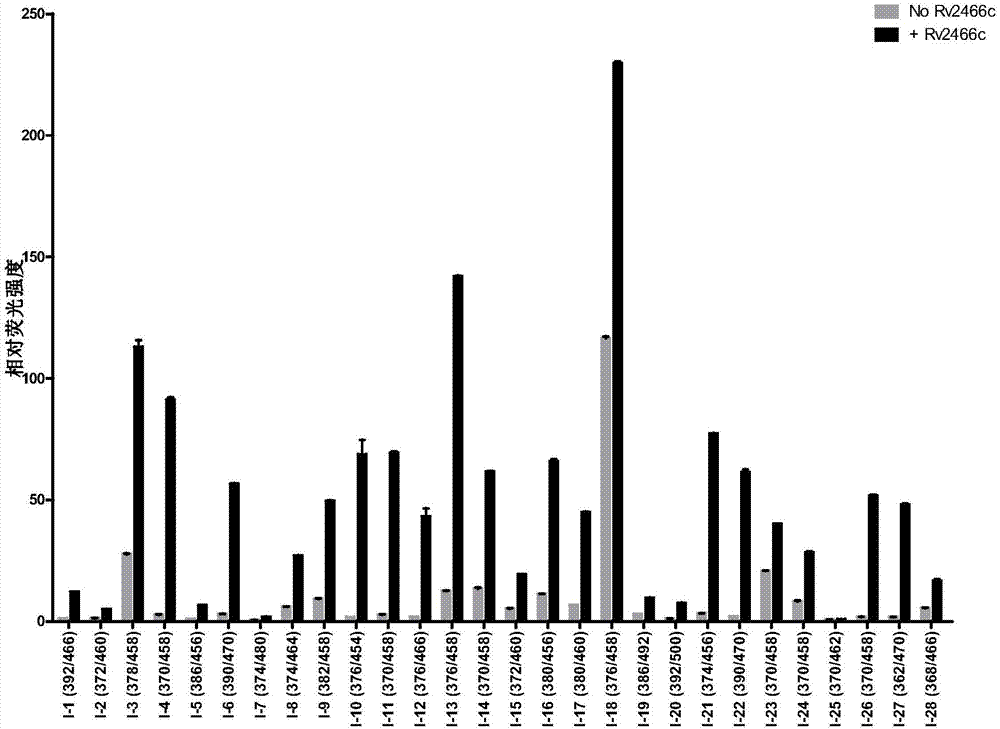
[0106] Fluorescence response of compound shown in embodiment 1 formula I to Rv2466c enzyme
[0107] Instrument: multifunctional microplate reader ( Flash, Thermo Scientific)
[0108] Reagents and materials: compound (I-1 to I-28, I-69), Rv2466c, MSH, Tris-Cl (pH 7.5), DMSO, 96-well microtiter plate (Corning, 3603)
[0109] Operation: 1) Prepare 50mM Tris-Cl (pH 7.5) buffer solution containing 0.5mM MSH, and put 20mL buffer solution into two sterile tubes. 2) Add a certain amount of Rv2466c to one of the tubes so that the final concentration is 200 μg / mL, which is the enzyme-containing working solution, and add the same amount of Tris-Cl buffer solution to the other tube as the enzyme-free control solution. 3) The compounds of the examples were respectively dissolved in DMSO to prepare a solution of 25 μg / mL, and 20 μL of each compound was added to four wells of a 96-well plate, two of which were used as a control group without enzymes, The other two wells are the enzyme-c...
Embodiment 2
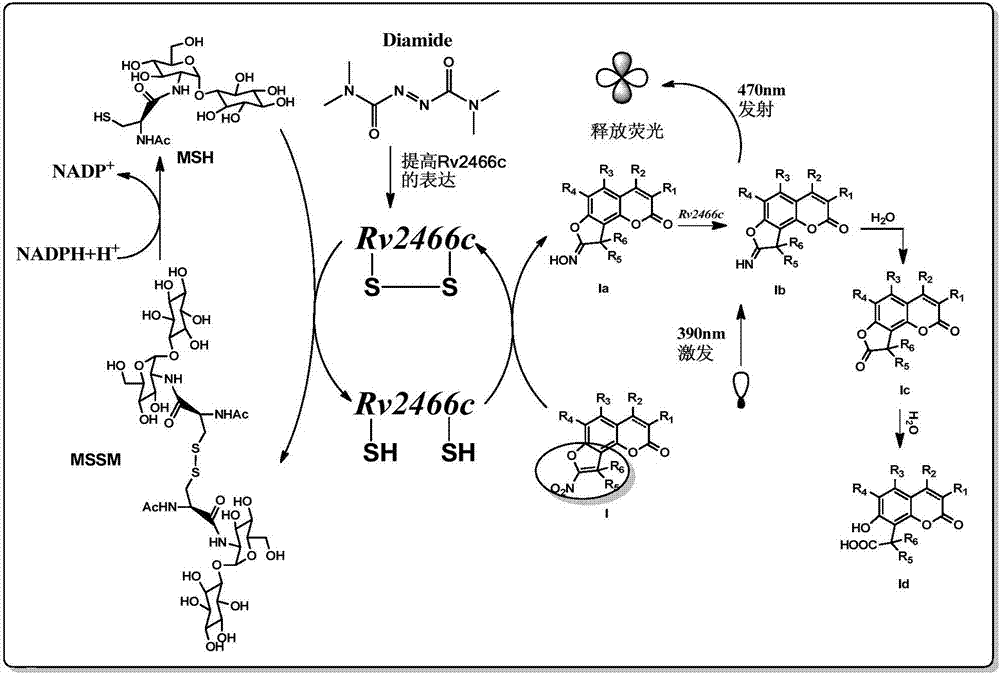
[0115] On the basis of Example 1, the inventor followed the steps below, taking compound I-6 as an example to study the fluorescence characteristics of compound I of the present invention and its nitro reduction product:
[0116] According to the results of mass spectrometry, compound I-6 is reduced to I-6a, I-6b and I-6d in the presence of Mtb, and intermediate I-6c is inferred from the structure of I-6d. Therefore, it is speculated that I -6 has the following reduction and hydrolysis processes:
[0117]
[0118] Then proceed as follows:
[0119] Instrument: multifunctional microplate reader ( Flash, Thermo Scientific)
[0120] Reagents and materials: Compounds (I-6, I-6a, I-6b, I-6c, I-6d), DMSO, 7H9 medium, 96-well enzyme plate (Corning, 3603)
[0121] Operation: 1) Take an appropriate amount of compound I-6, I-6a, I-6b, I-6c, and I-6d powder, and prepare it into a 10 mg / mL DMSO solution. 2) Dilute the DMSO solution 1000 times with 7H9 medium to obtain a test solut...
Embodiment 3
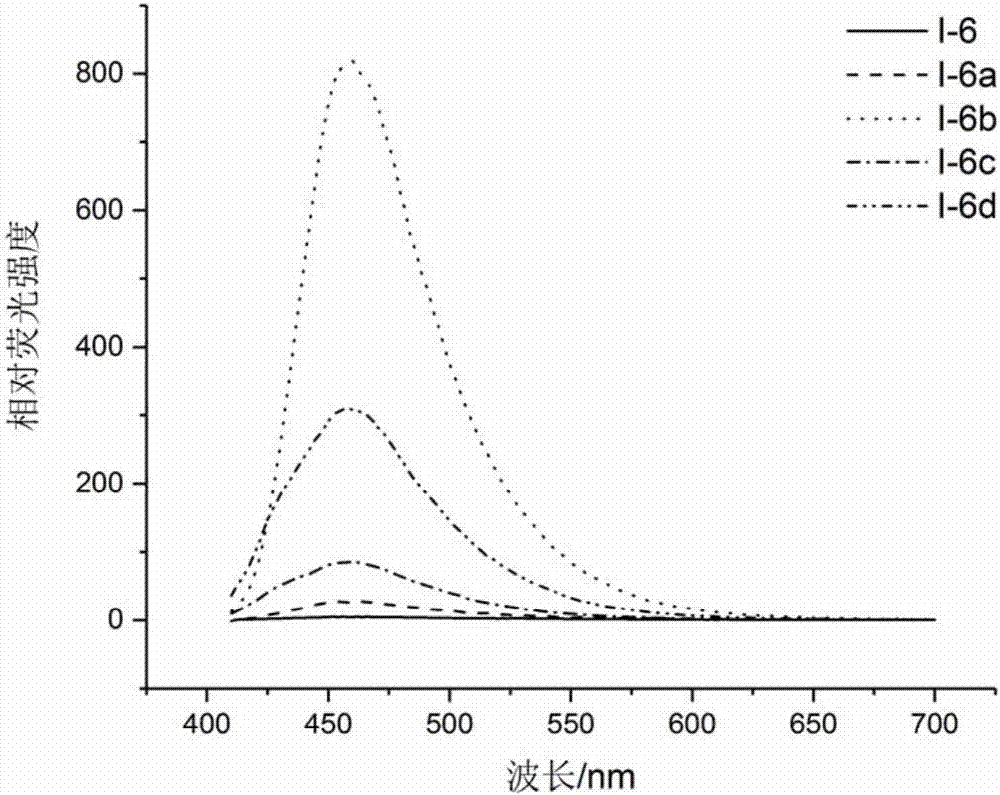
[0125] On the basis of Example 1-2, the inventor followed the steps below, taking compound I-6 as an example, to study the fluorescence response of compound I of the present invention to Rv2466c enzyme:
[0126] Instrument: multifunctional microplate reader ( Flash, Thermo Scientific)
[0127] Reagents and materials: compound I-6, Rv2466c, DTT (dithiothreitol), MSH, Tris-Cl (pH 7.5), DMSO, 96-well microtiter plate (Corning, 3603)
[0128] Operation: Steps 1)-4) are the same as in Example 2. In step 5), it is necessary to track and detect the fluorescence value under excitation at 390nm and emission at 470nm. Wherein, the reaction conditions are: 0.5 mM MSH, 1 mM DTT, 200 μg / mL Rv2466c and 2.5 μg / mL Example compound I-6.
[0129] see results Figure 4 .
[0130] Figure 4 In the presence of DTT and MSH, after Rv2466c catalyzed the reduction of compound I-6, the detection results were tracked under the conditions of excitation light and emission light (390 / 470nm). Thus, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com