Human alpha1-microglobulin (MG) determination kit with high sensitivity and wide detection range
A microglobulin and kit technology, applied in the field of immunodiagnosis, can solve the problems of not being able to detect serum, plasma and urine samples at the same time, the reagent sensitivity is not enough, and the sensitivity and linear range cannot be well considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
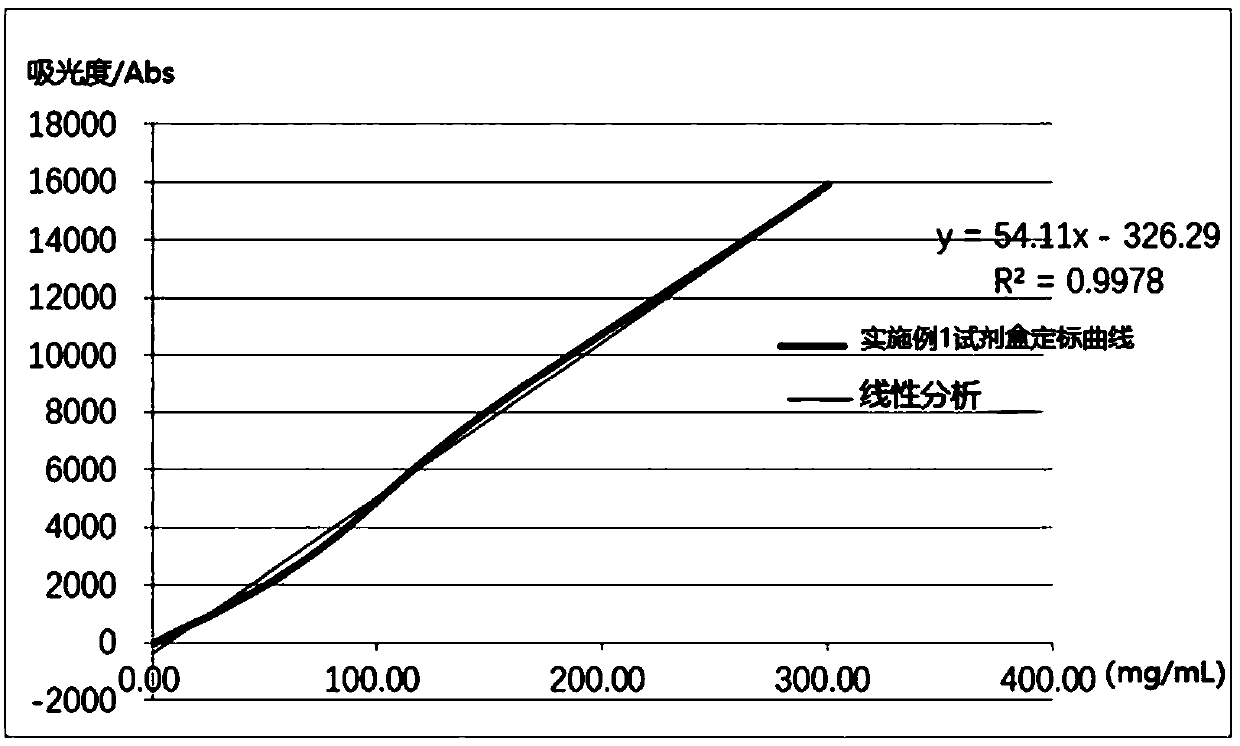
Embodiment 1
[0063] A human α1-microglobulin assay kit, comprising reagent 1, reagent 2 and human α1-MG calibrator with different gradient concentrations; wherein,
[0064] Reagent 1 included the following final concentrations of components: 50 mM PBS buffer (pH 7.4), 1.5% (w / v) BSA, 3% (w / v) PEG6000, 1.5% (w / v) Mw Be the dextran of 40000, the NaCl of 150mM, the Tween-20 of 0.05% (w / v), the Krovin300 of 0.1% (w / v), the EDTA of 2mM;
[0065] Reagent 2 includes the components of the following final concentrations: carboxyl latex microspheres (average particle diameter is 223nm, microsphere final concentration is 0.015% (w / v)), 0.003% (w / v) labeled human α1-MG monoclonal antibody, carboxylated latex microspheres (average particle diameter is 123nm, microsphere final concentration is 0.2% (w / v)), 0.045 % (w / v) labeled human α1-MG polyclonal antibody, 50mM PBS buffer (pH7.2), 100mM ammonium chloride, 1% (w / v) BSA, 5% (w / v) sucrose, 0.05% (w / v) Tween-20, 0.1% (w / v) ProClin300.
[0066] Calib...
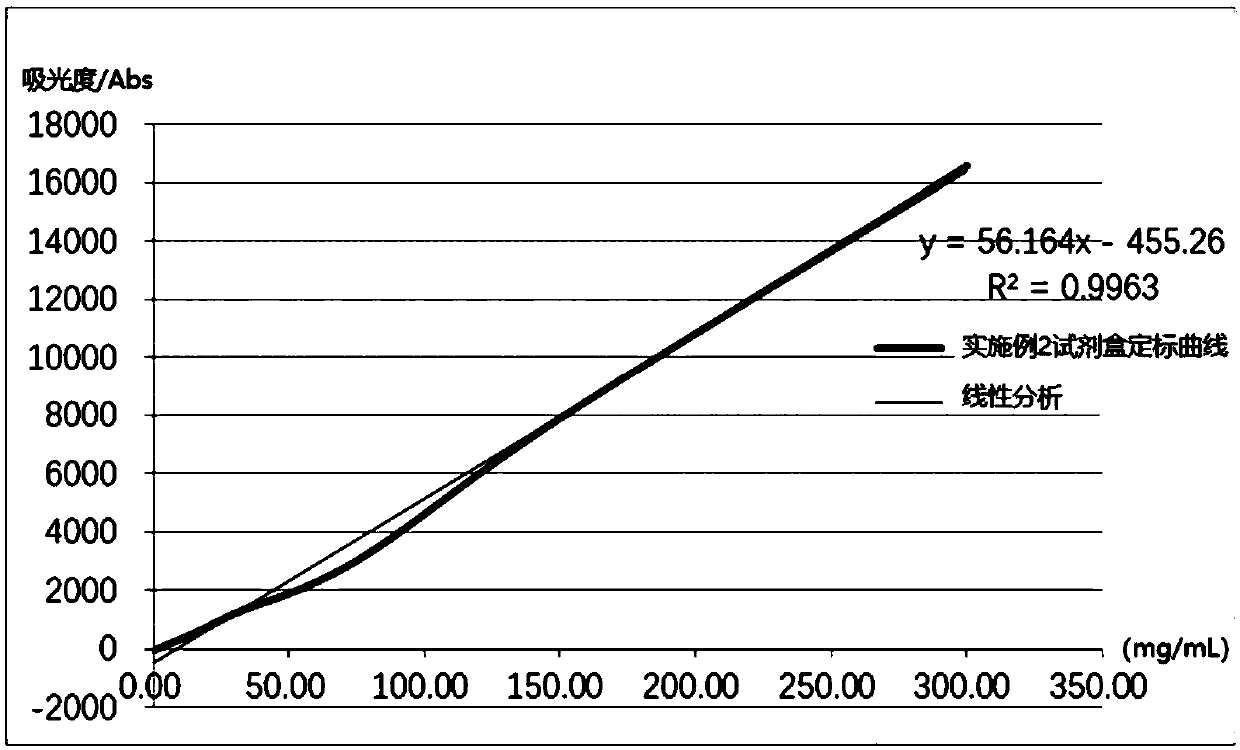
Embodiment 2
[0072] A human α1-microglobulin assay kit, comprising reagent 1, reagent 2 and human α1-MG calibrator with different gradient concentrations; wherein,
[0073] Reagent 1 included the following final concentrations of components: 20 mM MOPS buffer (pH 7.4), 0.5% (w / v) BSA, 2% (w / v) PEG8000, 150 mM NH 4 Cl, the Mw of 3% (w / v) is the polylysine of 5000, the Tween-20 of 0.05% (w / v), the Krovin500 of 0.1% (w / v), the EDTA of 5mM;
[0074] Reagent 2 includes the components of the following final concentrations: carboxyl latex microspheres (average particle diameter is 186nm, final concentration of microspheres is 0.02% (w / v)), 0.005% (w / v) of human α1-MG monoclonal antibody / v) labeled human α1-MG monoclonal antibody, carboxylated latex microspheres (average particle diameter is 88nm, microsphere final concentration is 0.2% (w / v)), 0.04 % (w / v) labeled human α1-MG polyclonal antibody, 50mM HEPES buffer (pH 7.4), 150mM potassium chloride, 0.1% (w / v) gelatin, 4% (w / v) sucrose, 0.5 % ...
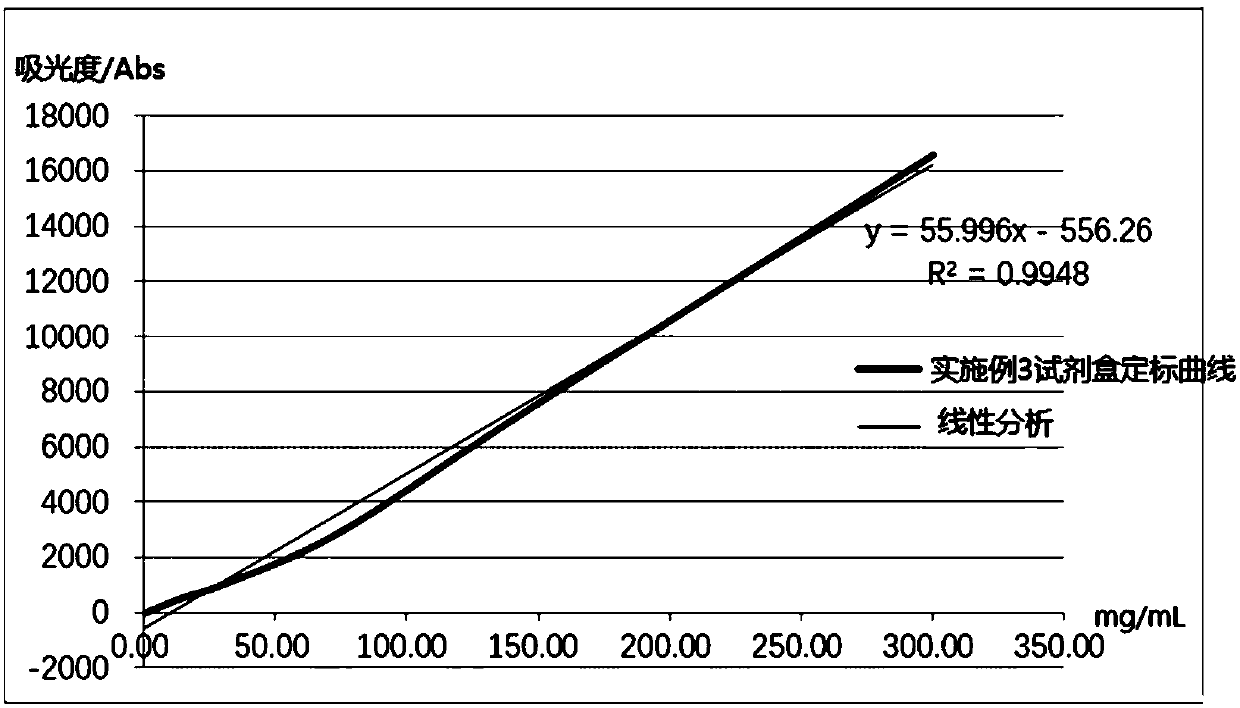
Embodiment 3
[0081] A human α1-microglobulin assay kit, comprising reagent 1, reagent 2 and human α1-MG calibrator with different gradient concentrations; wherein,
[0082] Reagent 1 includes the following final concentrations of components: 100 mM Tris-Cl buffer (pH 8.0), 0.25% (w / v) of BSA, 1% (w / v) of PEG20000, 0.5% (w / v) The Mw is 500000 dextran, the KCl of 50mM, the Tween-80 of 0.05% (w / v), the ProClin300 of 0.1% (w / v), the EDTA of 5mM;
[0083] Reagent 2 includes the following final concentration components: carboxyl latex microspheres (average particle diameter is 196nm, microsphere final concentration is 0.015% (w / v)), 0.007% (w / v) labeled human α1-MG monoclonal antibody, carboxyl latex microspheres (average particle diameter is 70nm, microsphere final concentration is 0.25% (w / v)), 0.06 % (w / v) labeled human α1-MG polyclonal antibody, 50mM MOPS (pH 7.2), 50mM magnesium chloride, 0.2% (w / v) gelatin, 0.2% (w / v) BSA, 3% (w / v) Sucrose, 1% (w / v) Glucose, 0.05% (w / v) Tween-20, 0.1% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com