Patents
Literature
184 results about "Guanidinium chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Guanidinium chloride or guanidine hydrochloride, usually abbreviated GuHCl and sometimes GdnHCl or GdmCl, is the hydrochloride salt of guanidine.
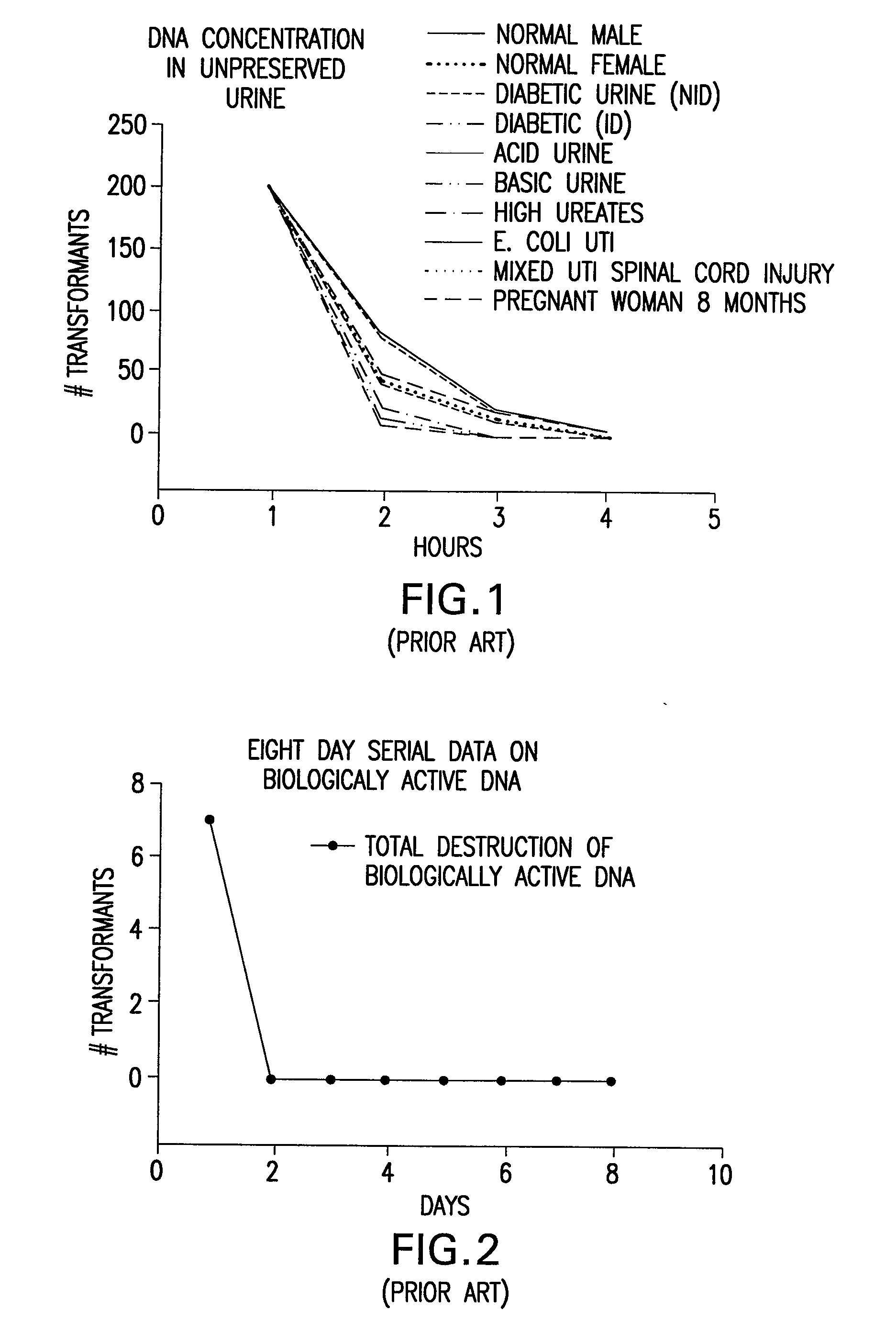
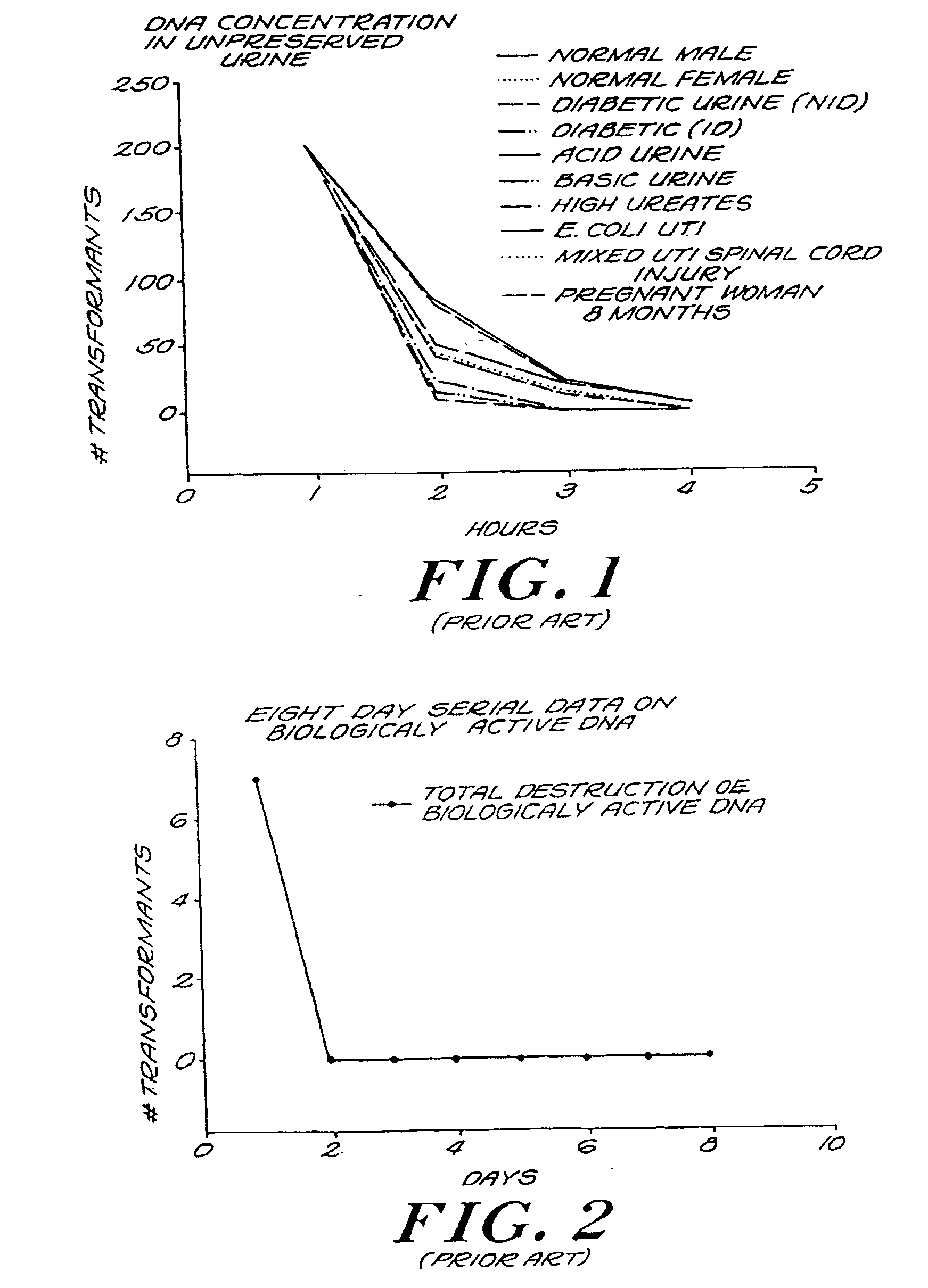
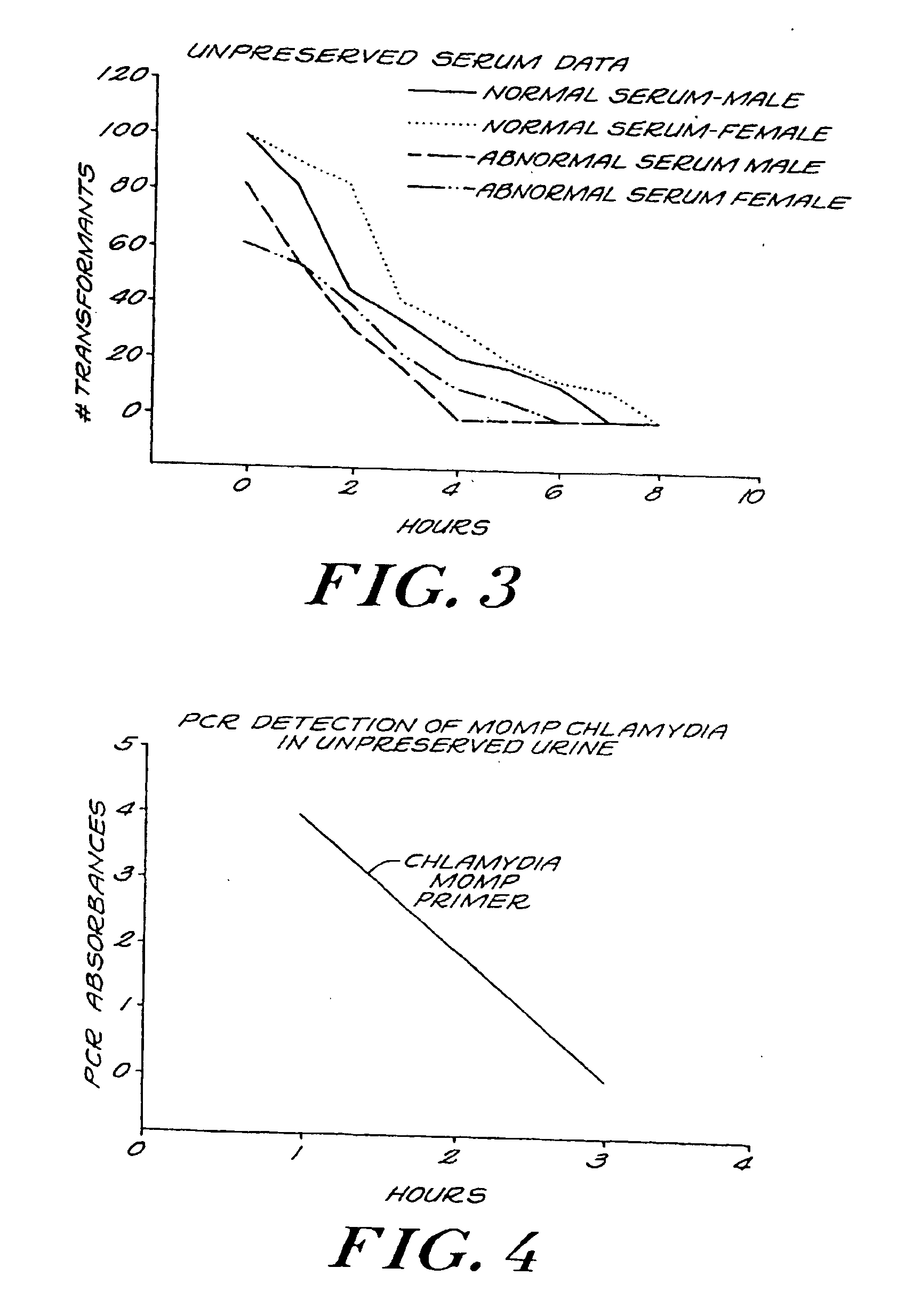
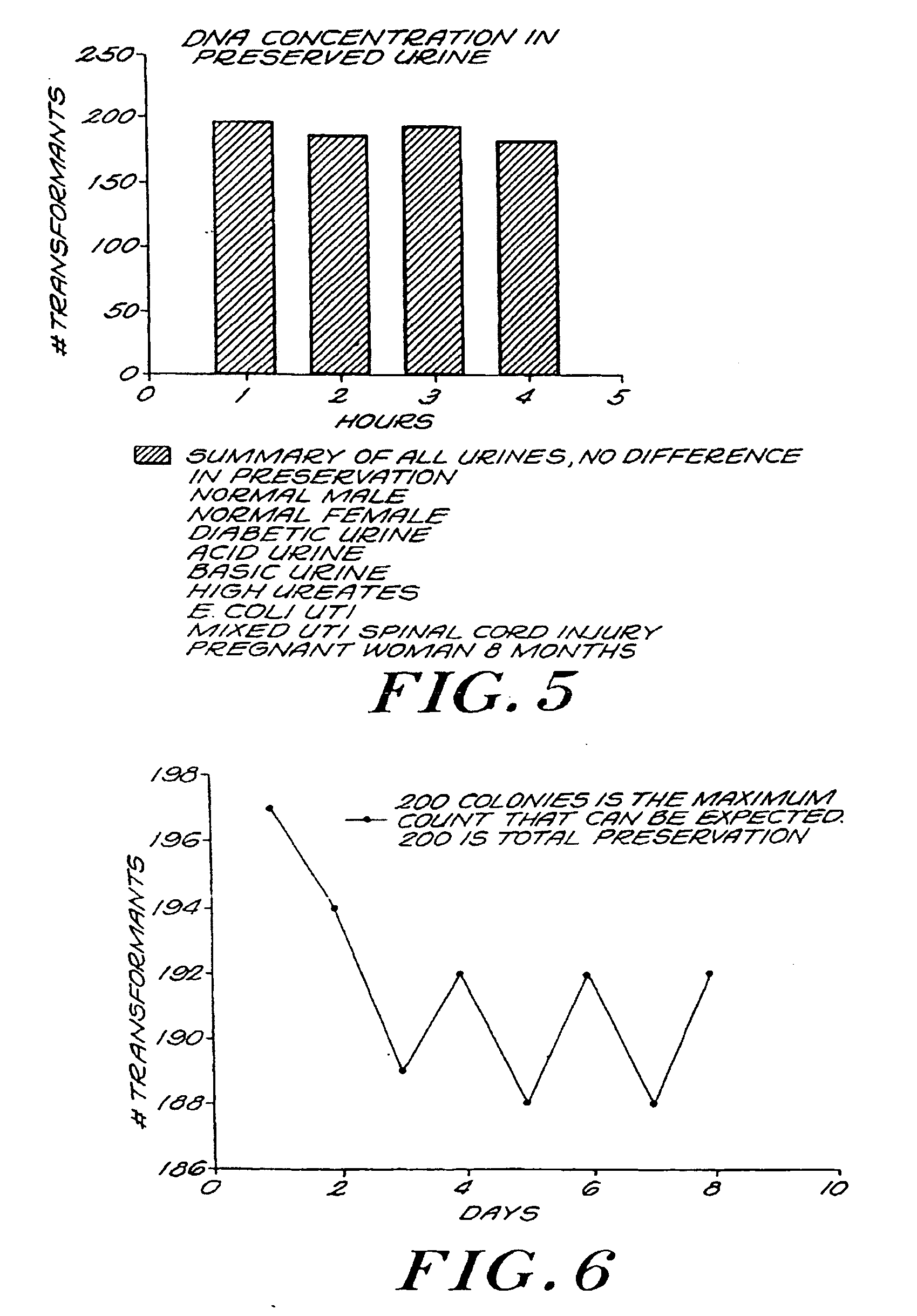
Urine Preservation System
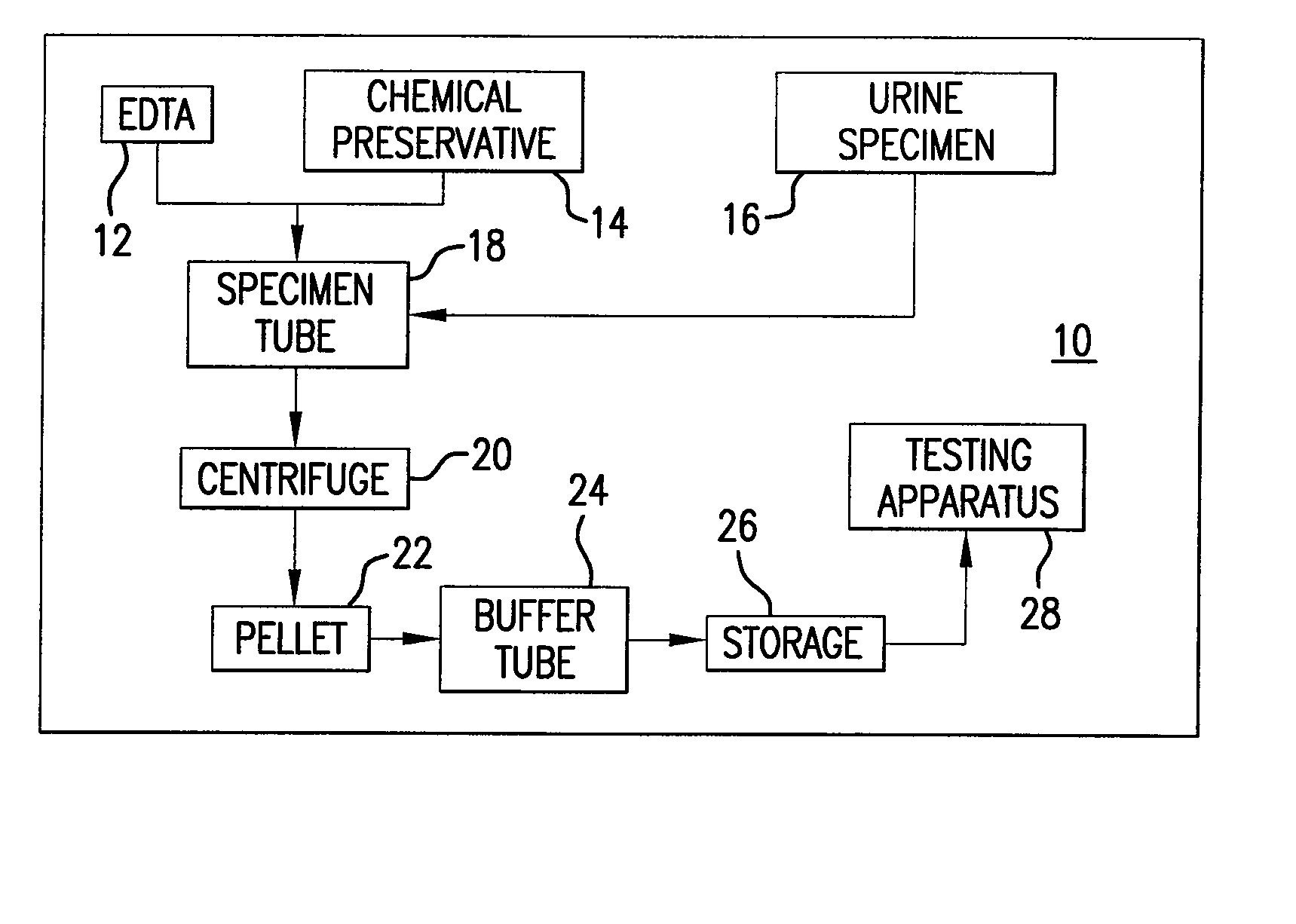
An improved method of preserving a molecule in a bodily fluid comprises: (1) providing a preservative solution comprising: (a) an amount of a divalent metal chelator selected from the group consisting of ethylenediaminetetraacetic acid (EDTA), (ethylenebis(oxyethylenenitrilo))tetraacetic acid (EGTA), and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and salts thereof in the range of from about 0.001 M to about 2 M; and (b) an amount of at least one chelator enhancing component selected from the group consisting of lithium chloride, guanidinium chloride, guanidinium thiocyanate, sodium salicylate, sodium perchlorate, and sodium thiocyanate in the range of from about 0.1 M to about 10 M; and (2) adding the preservative solution to the bodily fluid, thus preserving the molecule. The molecule can be a protein or a small molecule, such as a steroid. The invention also encompasses preservative compositions suitable for preserving proteins or small molecules, and kits. Preservative compositions can further include at least one enzyme inactivating component selected from the group consisting of manganese chloride, sarkosyl, and sodium dodecyl sulfate in the range of up to about 5% molar concentration. Compositions and methods according to the present invention have many diagnostic and forensic uses, in addition to being suitable for preparing compositions usable by hunters for attracting animals.
Owner:SIERRA MOLECULAR CORP
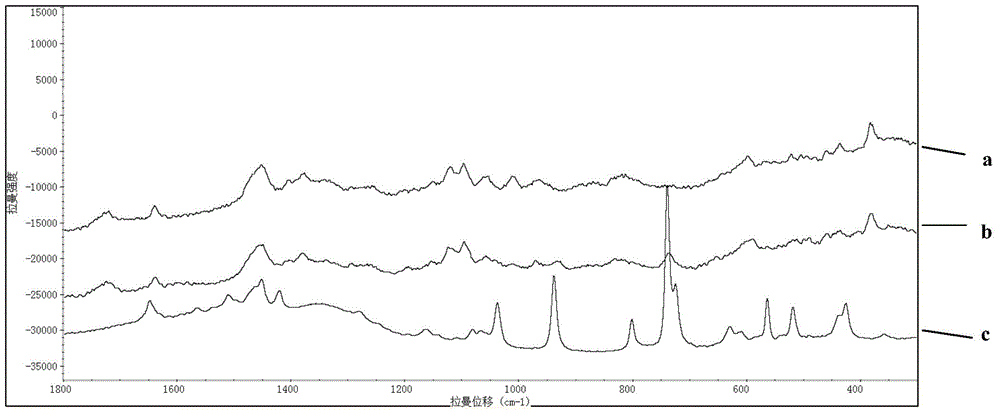
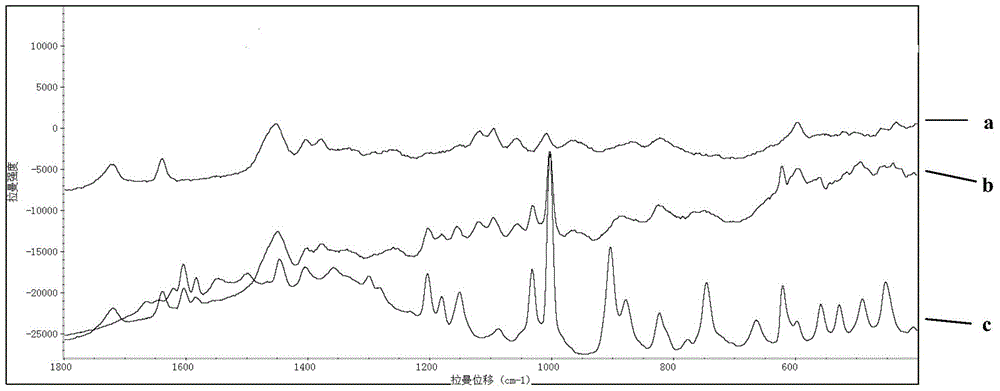
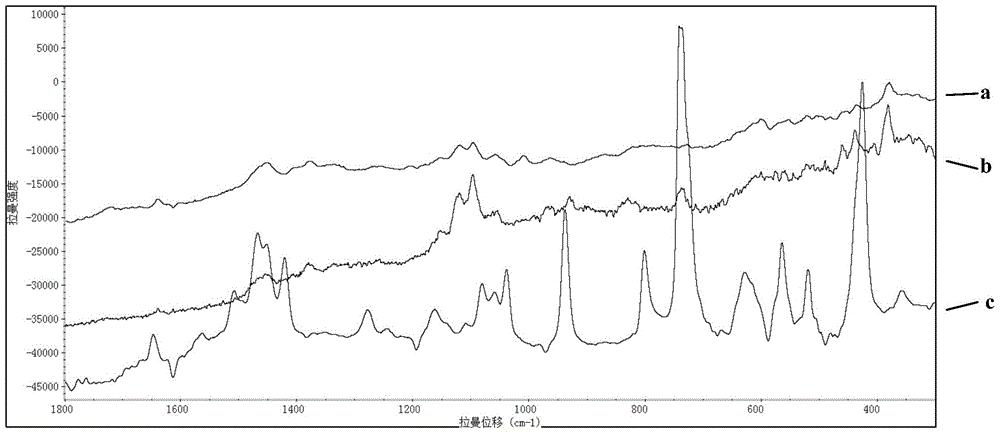
Rapid Raman spectrum detection method for molecularly imprinted membrane
InactiveCN104458695AAdsorptiveHigh strengthPreparing sample for investigationRaman scatteringFluorescenceSurface-enhanced Raman spectroscopy
The invention relates to a Raman spectrum detection method for a molecularly-imprinted membrane (MIM) by use of a Raman spectrometer used as a detection means with the combination of a molecular imprinting technology and a membrane technology and is used for rapidly detecting target molecules-dimethylguanidine hydrochloride and the like in real time. According to the method, a piece of lens paper for an optical lens is used as a substrate (carrier), guanidine hydrochloride or 4-(2-amino ethyl) benzsulfamide is used as an imprinted template, nanogold / silver sol is added in a pre-polymerization solution, and a polymer is directly light-gathered on the lens paper, so as to be convenient to carry and detect. The addition of the nanogold / silver sol is similar to the surface enhanced raman spectroscopy in principle, the response strength of the Raman spectrum can be enhanced and the fluorescence signal interference is reduced; the molecularly-imprinted membrane after and before target adsorption is compared with a standard substance, so that the peaked change in the Raman spectrum can be observed and whether a practical sample contains a target molecule can be judged.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
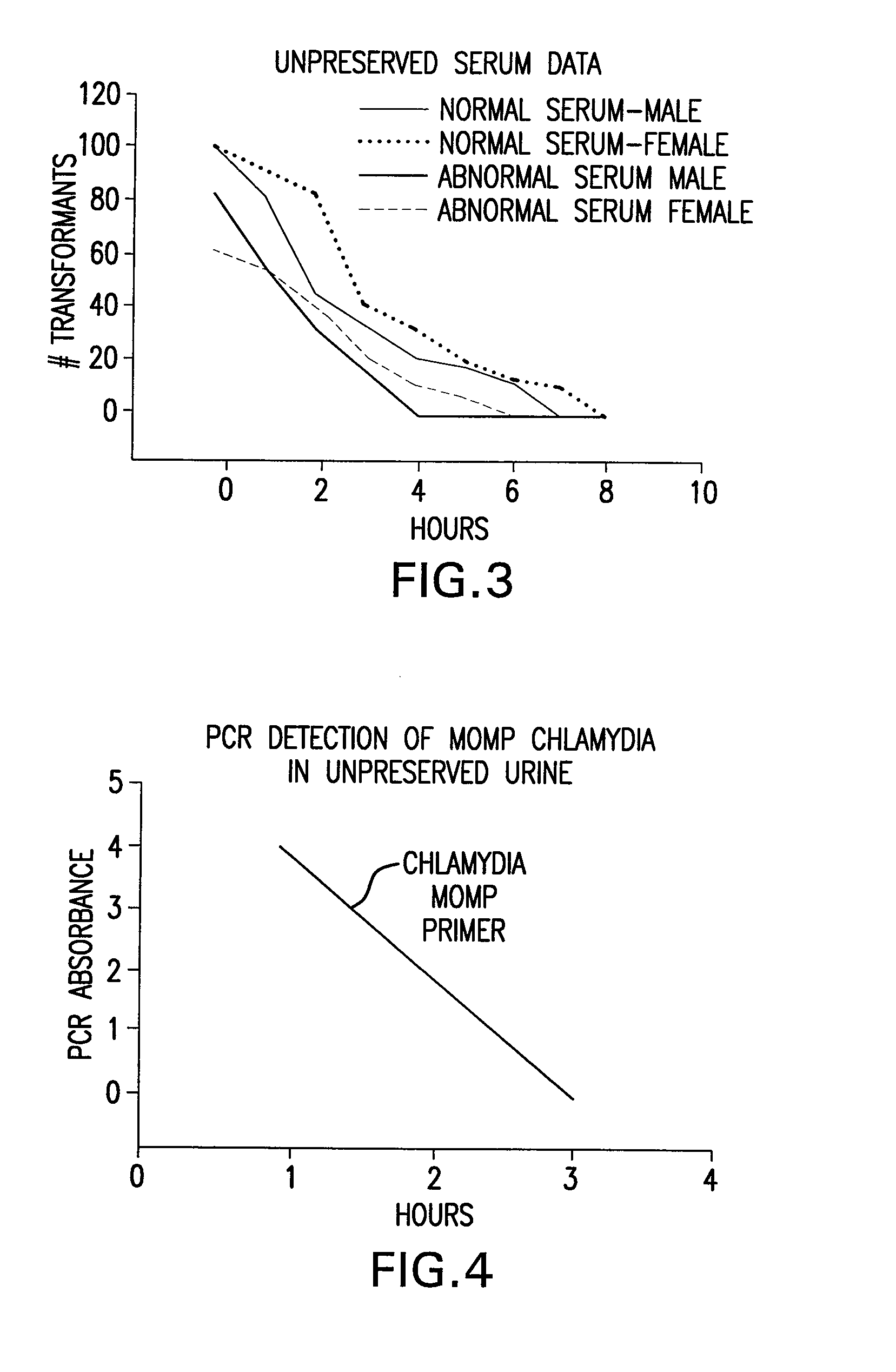
Urine preservation system
An improved method of preserving a molecule in a bodily fluid comprises: (1) providing a preservative solution comprising: (a) an amount of a divalent metal chelator selected from the group consisting of ethylenediaminetetraacetic acid (EDTA), (ethylenebis(oxyethylenenitrilo))tetraacetic acid (EGTA), and 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and salts thereof in the range of from about 0.001 M to about 2 M; and (b) an amount of at least one chelator enhancing component selected from the group consisting of lithium chloride, guanidinium chloride, guanidinium thiocyanate, sodium salicylate, sodium perchlorate, and sodium thiocyanate in the range of from about 0.1 M to about 10 M; and (2) adding the preservative solution to the bodily fluid, thus preserving the molecule. The molecule can be a protein or a small molecule, such as a steroid. The invention also encompasses preservative compositions suitable for preserving proteins or small molecules, and kits. Preservative compositions can further include at least one enzyme inactivating component selected from the group consisting of manganese chloride, sarkosyl, and sodium dodecyl sulfate in the range of up to about 5% molar concentration. Compositions and methods according to the present invention have many diagnostic and forensic uses, in addition to being suitable for preparing compositions usable by hunters for attracting animals.
Owner:SIERRA MOLECULAR CORP
Non-denatured II type collagen of animal cartilage source and preparation method for non-denatured II type collagen
InactiveCN105331662AGood degreasingEasy to handlePeptide preparation methodsFermentationCartilage cellsFreeze-drying
The invention discloses a preparation method for non-denatured II type collagen of an animal cartilage source. The preparation method is characterized by comprising the following steps: taking fresh and traceable animal cartilages as raw materials; carrying out purification treatment on the animal cartilages by virtue of processes of repeated freeze-thawing, ultrasonic degreasing, hypertonic solution decellularizing, acid-base softening and the like; carrying out processes of guanidine hydrochloride sugar-removing, pickling, compound enzyme treatment, multi-time salting-out, multi-time centrifugal purification, membrane dialysis, freeze-drying and the like, thereby obtaining spongy non-denatured II type collagen. The high-purity high-yield II type collagen obtained by the preparation method keeps the original complete triple-helix structure of the collagen, is high in biological activity, stable in structure, easy to store and beneficial to adhesion, growth and multiplication of cartilage cells, and can be used for preparing medical biological materials.
Owner:SICHUAN UNIV
Process for solubilization of recombinant proteins expressed as inclusion body
The present invention relates to the solubilization and recovery in high yield, of inclusion body proteins from host cells using an appropriate denaturating agent. The process avoids the use of high concentration of chaotropic agents such as guanidine hydrochloride or urea.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Cervical cell preservation and DNA fast extraction integrated kit and extraction method
ActiveCN105368817AGuaranteed accuracyImprove stabilityDead animal preservationDNA preparationA-DNABiology
The invention provides a cervical cell preservation and DNA fast extraction integrated kit which comprises cell preservation liquid, a first cleaning solution, a second cleaning solution, eluent and a DNA purification column. The cell preservation liquid comprises 2-3mol / L of guanidine hydrochloride or guanidinium thiocyanate, 5-50mmol / L of Tris-HCl, 1-10mmol / L of EDTA and 5-7.5v / v% of Trixon-100. The cervical cell preservation and DNA fast extraction integrated kit has the advantages that cervical sample DNA can be preserved in the cell preservation liquid of the kit for more than a week under room temperature, transportation of collected samples is facilitated, subsequent DNA extract does not need complex splitting anymore, the whole extraction process can be completed in 10 minutes, and DNA extraction efficiency is increased evidently; in addition, the extraction operation only needs a liquid-moving device and a centrifuge, instrument dependence is low, and the extracted DNA is high in purity.
Owner:GWP BIOTECHNOLOGIES INC
Osteogenic device and a method for preparing the device
InactiveUS7186811B2Improve storabilityBone-inducing factorOsteogenic factorCollagen iProtein composition
Owner:BIOACTIVE BONE SUBSTITUTE
Porous Orthopedic Materials Coated With Demineralized Bone Matrix
A biomaterial including a porous biocompatible structure having interconnected pores, wherein the pores have interior walls and are interconnected by passageways, the interior walls and passageways being coated with an osteoinductive aqueous demineralized bone extract solution, the aqueous demineralized bone extract solution including growth factors, proteins, a demineralized bone matrix and at least one of a weak acid and a guanidine hydrochloride, wherein the demineralized bone matrix is present per 100 g of the solution in an amount of from about 2 g to about 10 g.
Owner:BIOMET MFG CORP
Construction method of medical titanium alloy implant surface growth factor delivery system
InactiveCN101791439ASimple preparation processGood adhesionProsthesisFreeze-dryingPolymer thin films
The invention relates to a construction method of a medical titanium alloy implant surface growth factor delivery system, which comprises the following steps that: micro-groove ridges are prepared on the surface of a medical low-elastic Beta titanium alloy; gelatin is dissolved into deionized water, and then 2-imine hydrogen chloride mercaptan is added to be dialyzed and dried, to prepare SH gelatin; the SH gelatin is dissolved in double-distilled water to be ultrasonically homogenized to prepare B liquid, emulsifier is added into liquid paraffin, and then the B liquid is dripped into the liquid paraffin which is added with the emulsifier, isopropyl alcohol and ether are cleaned, added with glutaraldehyde and cured, ether elutes, dries and sieves out the cured substance, and 60Co irradiates and sterilizes, to prepare SH gelatin microspheres; and the microspheres are soaked into rhBMP2 guanidine hydrochloride saturated solution and freeze dried under a vacuum condition, to prepare the SH gelatin microspheres loaded with growth factors. Medical low-elastic Beta titanium alloy implant material is soaked into dopamine solution, and a layer of adhered polymer thin film is generated on the surface of the titanium alloy; and the implant material which decorates the surface is put into the growth factor SH gelatin microsphere solution, vacuum is pumped, and the medical titanium alloy implant surface growth factor delivery system is prepared.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Biological slime inhibitor and preparation method thereof
ActiveCN103535369AImprove the bactericidal effectHigh bactericidal activityBiocideDisinfectants1,3-PropanediolKetone
The invention discloses a biological slime inhibitor. The biological slime inhibitor is characterized by comprising the following components by weight percent: 5-30% of poly-hexylidene triamine guanidinium chloride, 1-10% of isothiazolinone, 1-10% of organic bromide, 5-40% of methanol solvent, and the balance of water, wherein the weight-average molecular weight of the poly-hexylidene triamine guanidinium chloride is Mw=10000-20000; the molecular weight distribution D is 1.0-1.1; the isothiazolinone is a mixture of 5-chloro-2-methyl-4-isothiazolin-3-ketone and 2-methyl-4-isothiazolin-3-ketone, of which the mass ratio is (2.5-4):1; the organic bromide is one or more of 2,2-dibromo-3-nitrogen propionamide, 2-brome-2-nitro-1,3-propanediol and 2,2-dibromo-2-nitroethanol. In addition, the invention also provides a preparation method of the biological slime inhibitor. The biological slime inhibitor disclosed by the invention has an extremely efficient sterilization effect and excellent alga-killing property, and has the characteristics of being long in bacteriostatic time, free of drug resistance, free of a bubble, safe to use and the like.
Owner:CNOOC TIANJIN CHEM RES & DESIGN INST +1
Process for solubilization of recombinant proteins expressed as inclusion body
The present invention relates to the solubilization and recovery in high yield, of inclusion body proteins from host cells using an appropriate denaturating agent. The process avoids the use of high concentration of chaotropic agents such as guanidine hydrochloride or urea.
Owner:NATIONAL INSTUTUTE OF IMMUNOLOGY
Preparation method of spherical N-doped C-coated metal oxide negative electrode material with multi-stage structure
ActiveCN108258223AUniform particle sizeStable structureCell electrodesSecondary cellsSolventCrystallinity
The invention discloses a preparation method of a spherical N-doped C-coated metal oxide negative electrode material with a multi-stage structure and belongs to the field of lithium ion batteries. Thepreparation method comprises the following specific steps: mixing and stirring N-methylpyrrolidone, polyvinylidene fluoride and guanidine hydrochloride to transparent gel, drying to remove a solventand calcining in argon to obtain N-doped C; dissolving sodium molybdate, nickel nitrate, ammonium fluoride and urea in an alcohol solution, stirring into a homogeneous solution and performing hydrothermal reaction; then mixing the N-doped C and the homogeneous solution, ball-milling and calcining to obtain a NiO / NiMoO4@N-C material; putting the NiO / NiMoO4@N-C material in distilled water and addingsodium dodecyl sulfate after ultrasonic treatment; adding pyrrole and hydrochloric acid, stirring, then adding an initiating agent, centrifuging, washing and drying to obtain. The negative electrodematerial synthesized by the preparation method disclosed by the invention is uniform and consistent in particle, good in dispersibility and high in degree of crystallinity and has the stable multi-stage composite structure, thereby having appreciable wide potential window reversible capacity, excellent rate capability and stable cycle life.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
4'-pyridylpyrimidine compound and synthetic method and application thereof
InactiveCN107434801AImprove practicalityOrganic chemistryFluorescence/phosphorescenceFluorescenceTurpentine
The invention discloses a 4'-pyridylpyrimidine compound and a synthetic method and application thereof. By using a derivative iso-longitolanone of natural renewable resource turpentine as a raw material, a novel 4'-pyridylpyrimidine compound is prepared. Iso-longitolanone and 4-pyridylaldehyde are subjected to condensation to generate 7-(pyridine-4'-yl-methylene)iso-longitolanone; 7-(pyridine-4'-yl-methylene)iso-longitolanone and guanidine hydrochloride are subjected to condensation and cyclization to obtain a 4'-pyridylpyrimidine compound 6,6,10,10-tetramethyl-4-(pyridine-4'-yl)-5,7,8,9,10,10a-hexahydro-6H-6a,9-methanobenzo-2-quinazolinamine. The compound can specifically recognize copper ion, can be specifically complexed with Cu<2+> ion and generates quenching of blue fluorescence. Thus, the compound can be used as a fluorescence probe for detection of copper ion and has good practicality.
Owner:NANJING FORESTRY UNIV
Water-treated polyhexamethylene guanidine hydrochloride sterilizing agent and preparation method thereof
InactiveCN104397006ANo pollution in the processNo corrosionBiocideFungicidesBiotechnologyIron sulfate
The application discloses a water-treated polyhexamethylene guanidine hydrochloride sterilizing agent and a preparation method thereof. The water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is prepared by weighing and uniformly mixing guanidine hydrochloride, hexamethylene diamine, hexamethylenediamine, polyethylene glycol, water, methylbenzene, a polymerization inhibitor, sulphonic acid, maleic anhydride, N-t-butyloxycarboryl-1,6-diamino hexane, a dispersing agent, ferric sulfate, methacrylic acid, dodecyl dimethyl benzyl ammonium chloride and methyl acrylate according to parts by weight. The water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is 100% soluble in water, has no volatility, can be completely neutralized with soaps, and has no pollution to environment; the integral of an acute skin irritancy test is 0, and the water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is colorless, non-poisonous, odorless and tasteless, and has no corrosion to metal, rubber and plastic; the sterilizing rate to escherichia coli, staphylococcus aureus, candida albicans, neisseria gonorrhoeae, penicillium, aspergillus, saprophytic bacteria and salmonella is 100%, when the water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is placed at 50-60 DEG C for a month, the sterilizing rate is 98-100%, when the water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is placed at 40-50 DEG C for half a year, the sterilizing rate is 97.5-99.5%, and when the water-treated polyhexamethylene guanidine hydrochloride sterilizing agent is placed at the room temperature for 1-2 years, the sterilizing rate is 95-99%.
Owner:SUZHOU YOUJUN ENVIRONMENTAL SCI & TECH
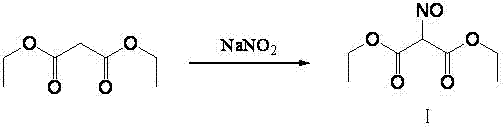
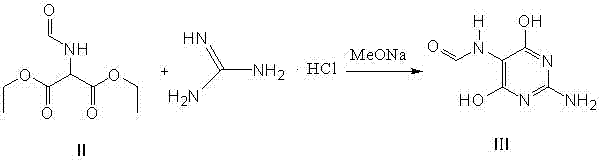
Method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Preparation method of 1, 3-diaminoguanidine monohydrochloride
ActiveCN104370776AReduce manufacturing costIncrease production profitOrganic chemistryPhosphatesDistillationDiaminoguanidine
Owner:甘肃汇能生物工程有限公司
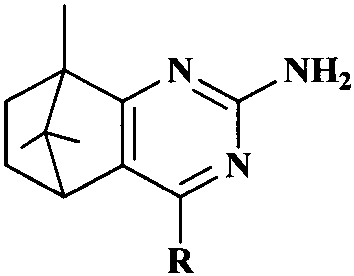
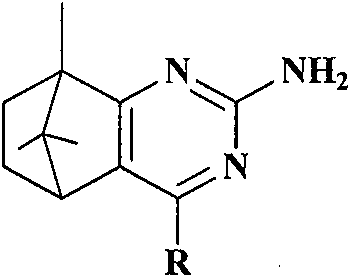
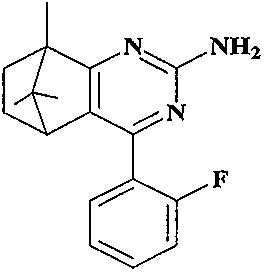
Synthesis and anti-tumor activity of camphoryl pyrimidines
InactiveCN110551070ARich sourcesEase of industrial productionOrganic chemistryAntineoplastic agentsAbnormal tissue growthProguanil Hydrochloride
The invention discloses camphoryl pyrimidines, and a preparation method and an anti-tumor activity study thereof. With camphor as a raw material and under an alkaline catalysis condition, a series ofalpha,beta-unsaturated ketones are obtained through aldol condensation reaction of camphor with different aromatic aldehydes respectively, a series of camphoryl pyrimidines are obtained through annulation reaction of alpha,beta-unsaturated ketones with guanidine hydrochloride through catalysis of potassium tert-butoxide, and the anti-tumor activity of the synthesized camphoryl pyrimidines is studied. Experiments show that the camphoryl pyrimidines have good inhibitory activity on human multiple myeloma cells (RPMI-8226), human breast cancer cells (MDA-MB-231) and human non-small cell lung cancer cells (A549), have less toxicity on normal cell human gastric mucosa cells (GES-1), and have potential anti-tumor application value.
Owner:NANJING FORESTRY UNIV
Immunodiagnostic assays using reducing agents
InactiveUS20060263854A1Prevent and treat HCV infectionBioreactor/fermenter combinationsFungiCell lysatesCell
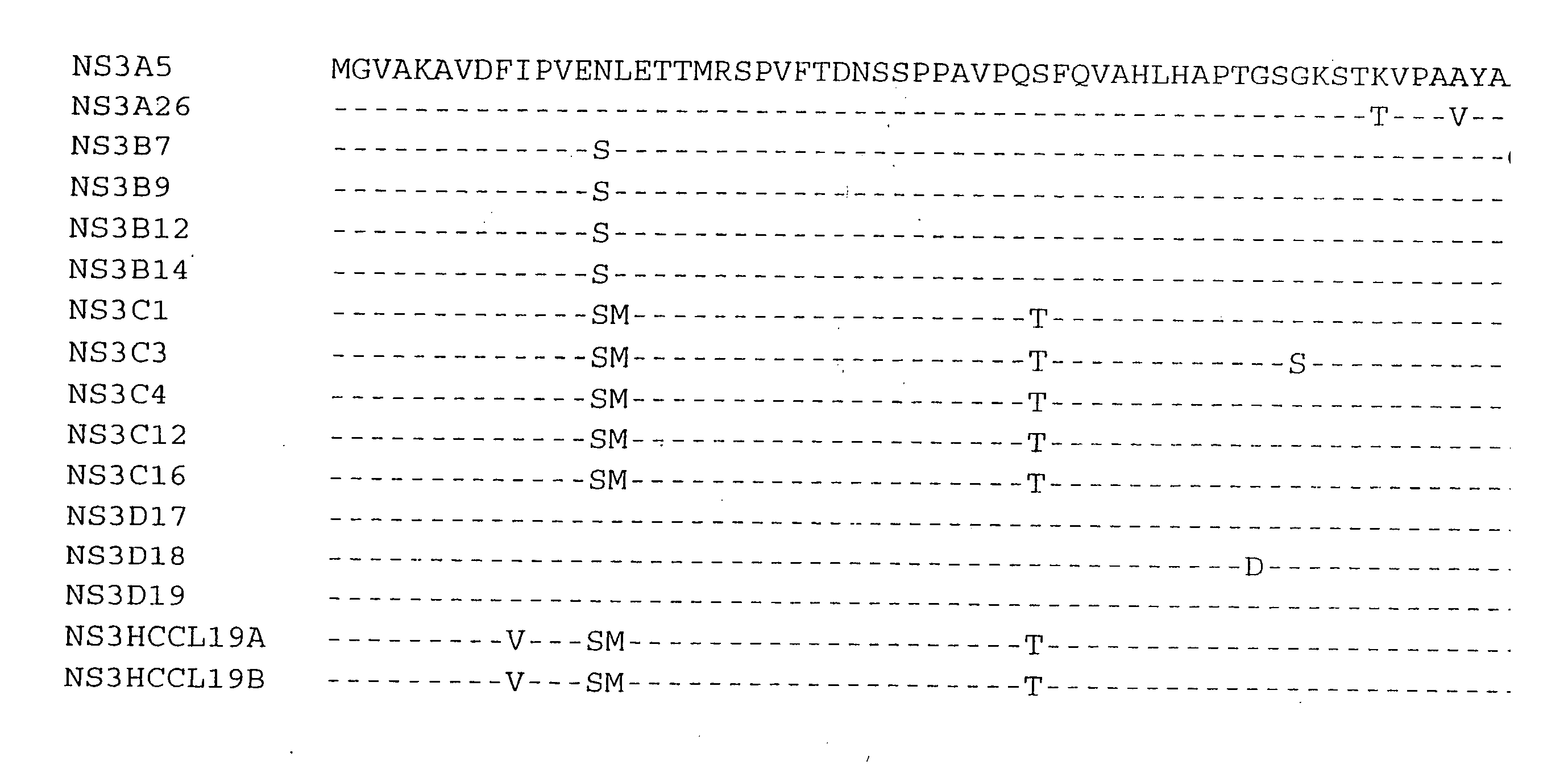
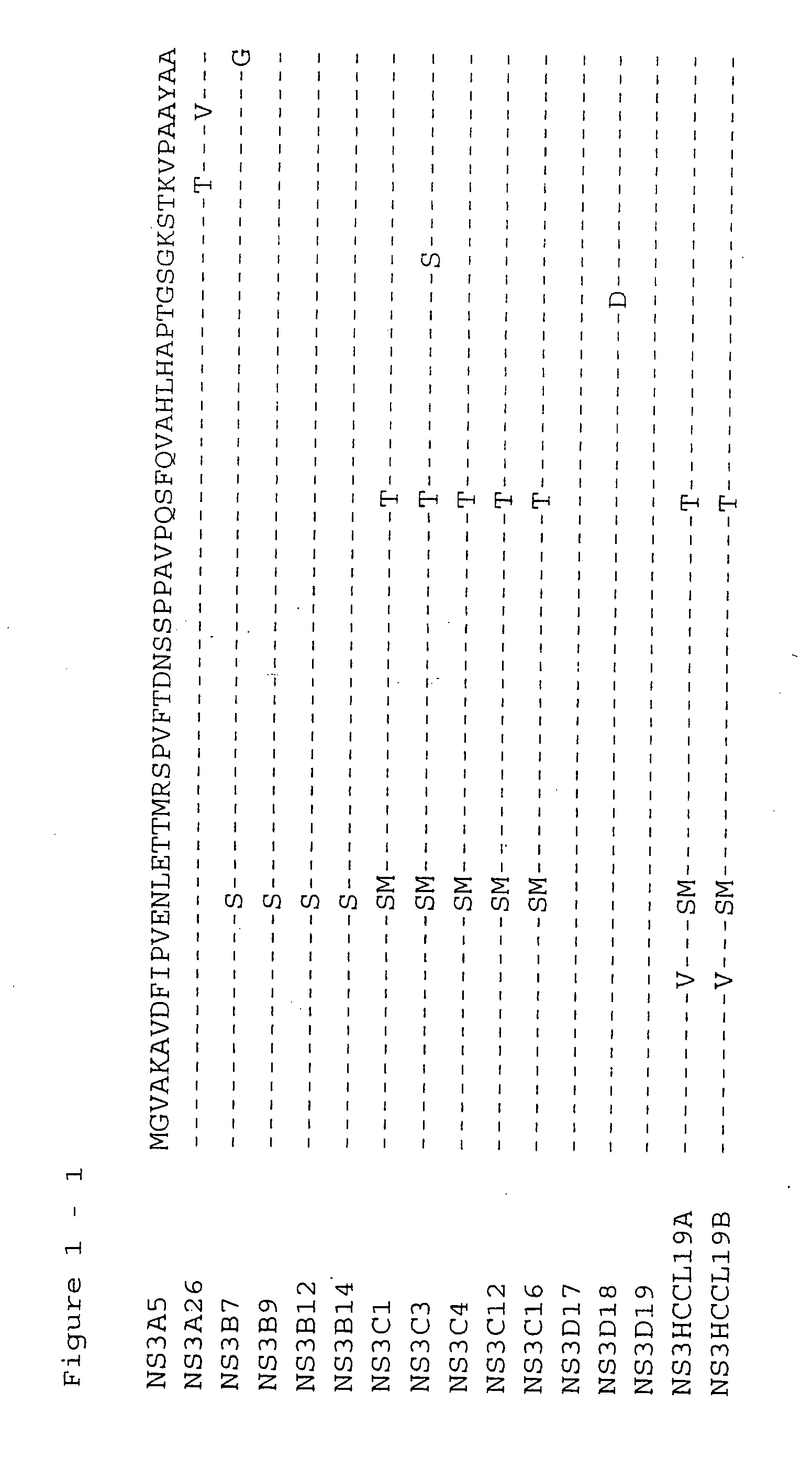
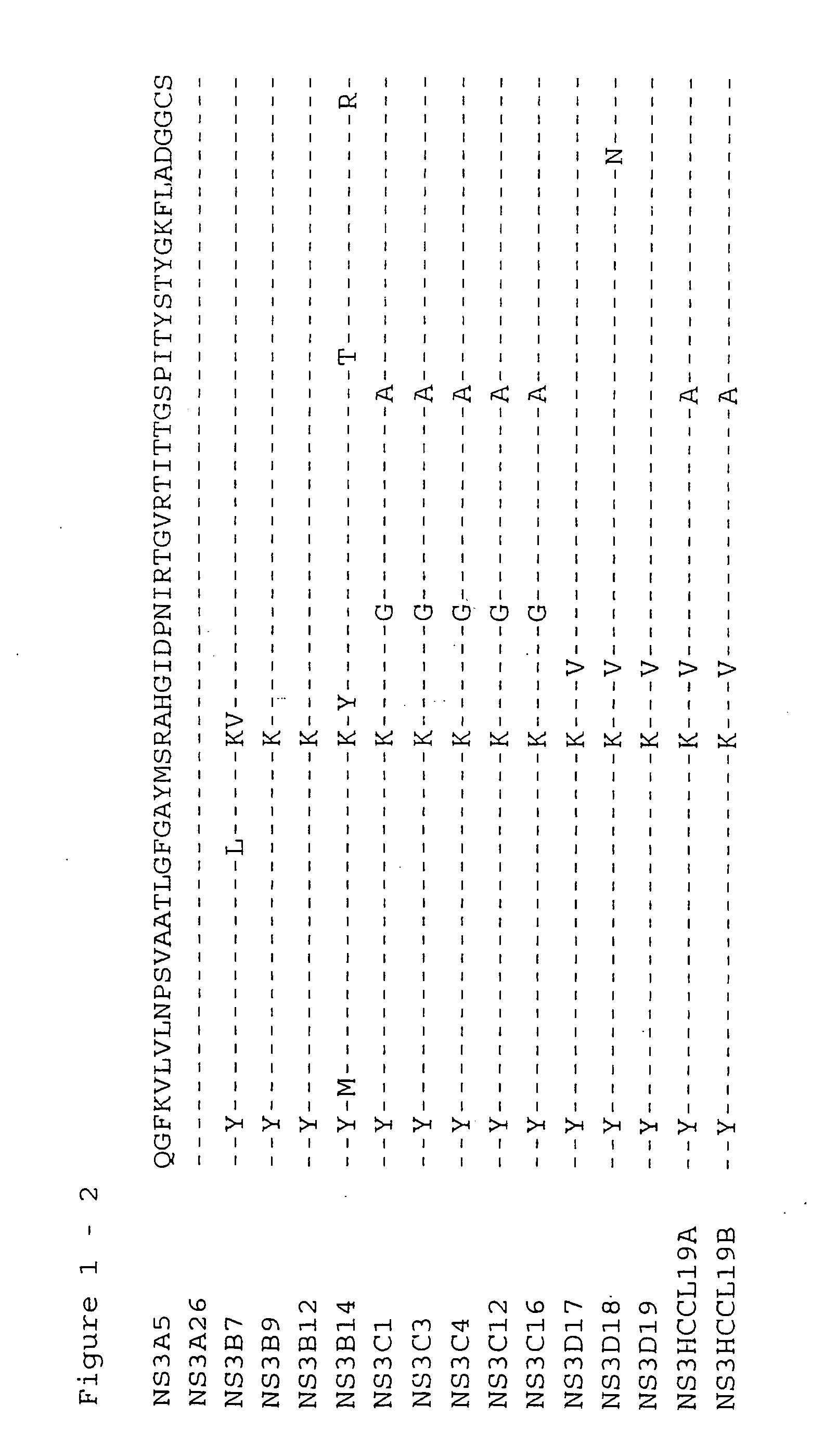
The present invention relates to a solid phase immunoassay comprising on said solid phase an antigen in the presence of a reducing agent. The present invention also relates to a method for purifying a cysteine containing recombinantly expressed protein comprising at least 2, preferably 3 or 4 and even more preferably all of the following steps: (a) sulphonation of a lysate from recombinant host cells or lysis of recombinant host cells in the presence of guanidinium chloride followed by a subsequent sulphonation of the cell lysate, (b) treatment with a zwitterionic detergent, preferably after removal of the cell debris, (c) purification of the sulphonated version of the recombinant protein or purification of the sulphonated version of the recombinant protein with subsequent removal of the zwitterionic detergent, with said purification being preferably chromatography, more preferably a Ni-IMAC chromatography with said recombinant protein being a His-tagged recombinant protein, (d) desulphonation of the sulphonated version of the recombinant protein, preferably with a molar excess of DTT, (e) storage in the presence of a molar excess of DTT. The present invention also relates to novel HCV NS3 sequences as depicted in FIGS. 1-8.
Owner:INNOGENETICS NV
Novel wood floor adhesive
InactiveCN104497895AImprove adhesionNot easy to fall offNon-macromolecular adhesive additivesMacromolecular adhesive additivesSodium acetateAdhesive cement
The invention discloses a novel wood floor adhesive. The adhesive is prepared by using the following raw materials, by weight, 5-10 parts of sodium polyacrylate, 0.5-1.7 parts of titanium dioxide, 1.5-3 parts of ethyl alpha-cyanoacrylate, 2.5-4.3 parts of alkyl polyglycoside, 1.5-2.8 parts of steam-exploded lignin, 1.2-3.6 parts of polyethylidene maleic acid, 0.5-1.2 parts of sodium diacetate and 0.9-2.2 parts of guanidine hydrochloride. Compared with present adhesives, the adhesive disclosed in the invention has the advantages of strong adhesion, no shedding, easily available raw materials, simple preparation technology, no benzene or aldehyde, non-toxicity, harmlessness, environmental protection, low carbon, high solid content, small shrinkage, good initial tack, fast drying speed, freeze resistance and easy gluing.
Owner:QINGDAO LAOXIANG TEA PROD
Nitrogen-modified catalyst applied to preparation of vinyl chloride and preparation method of nitrogen-modified catalyst
ActiveCN104289254ASimple preparation processLow costPreparation by hydrogen halide split-offOrganic-compounds/hydrides/coordination-complexes catalystsChemistryActivated carbon
The invention discloses a nitrogen-modified catalyst applied to preparation of vinyl chloride and a preparation method of the nitrogen-modified catalyst. According to the nitrogen-modified catalyst, active carbon is used as a carrier, wherein the nitrogen-modified catalyst comprises a metal salt loaded compound and a nitrogen-containing compound; according to the total mass of the catalyst, the mass percentage of the metal salt compound is 0.01-10%, and the mass percentage of the nitrogen-containing compound is 0.01-10%. The metal salt compound is strontium salt or barium salt or mixture of the strontium salt and the barium salt; the nitrogen-containing compound is selected from at least one of guanidine hydrochloride, acetamidine hydrochloride, acrylamide, urea, methanesulfonamide and cyanoacetamide. The nitrogen-modified catalyst is applied to reaction of preparing vinyl chloride by catalytic cracking of 1,2-dichloroethane; not only is the cracking temperature reduced, and also the nitrogen-modified catalyst is high in conversion rate of 1,2-dichloroethane and selectivity of vinyl chloride, and is efficient, and has energy-saving and environment-friendly effects.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Kit for extracting blood free DNA
PendingCN110904097AImprove extraction efficiencyImprove recycling efficiencyDNA preparationGuanidine isothiocyanateLysis
The invention discloses a kit for extracting blood free DNA. The kit of the invention includes a lysis solution, a binding solution, a cleaning solution 1 and a cleaning solution 2. The binding solution contains 2-5 M guanidine isothiocyanate or guanidine hydrochloride, 10-100mM Tris-HC1, 10-50mM EDTA, 5-15% Triton X-100, and 20-50% absolute ethanol or isopropanol. The cleaning solution 1 contains2-5 M guanidine isothiocyanate or guanidine hydrochloride, 10-100mM Tris-HCl, 10-50mM EDTA, 5-10% Triton X-100, SDS or Tween 20, and 20-50% absolute ethanol or isopropanol. The kit of the invention improves the binding solution and increases the extraction efficiency, improves the cleaning solution 1, increases the binding efficiency of the magnetic beads cfDNA and removes impurities, improves the recovery efficiency and purity of the cfDNA and reduces the material cost.
Owner:SHENZHEN HAPLOX BIOTECH
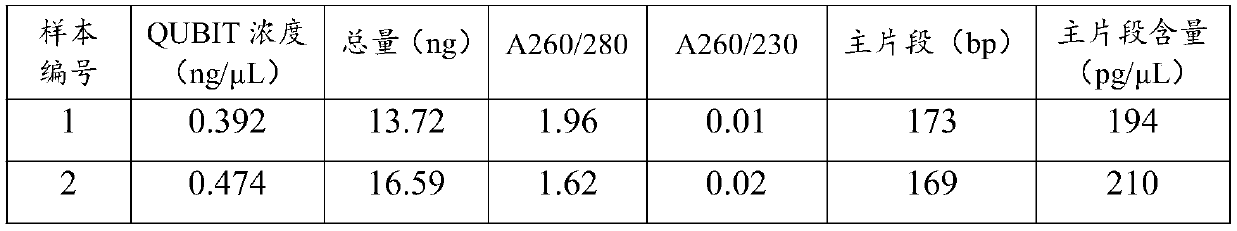
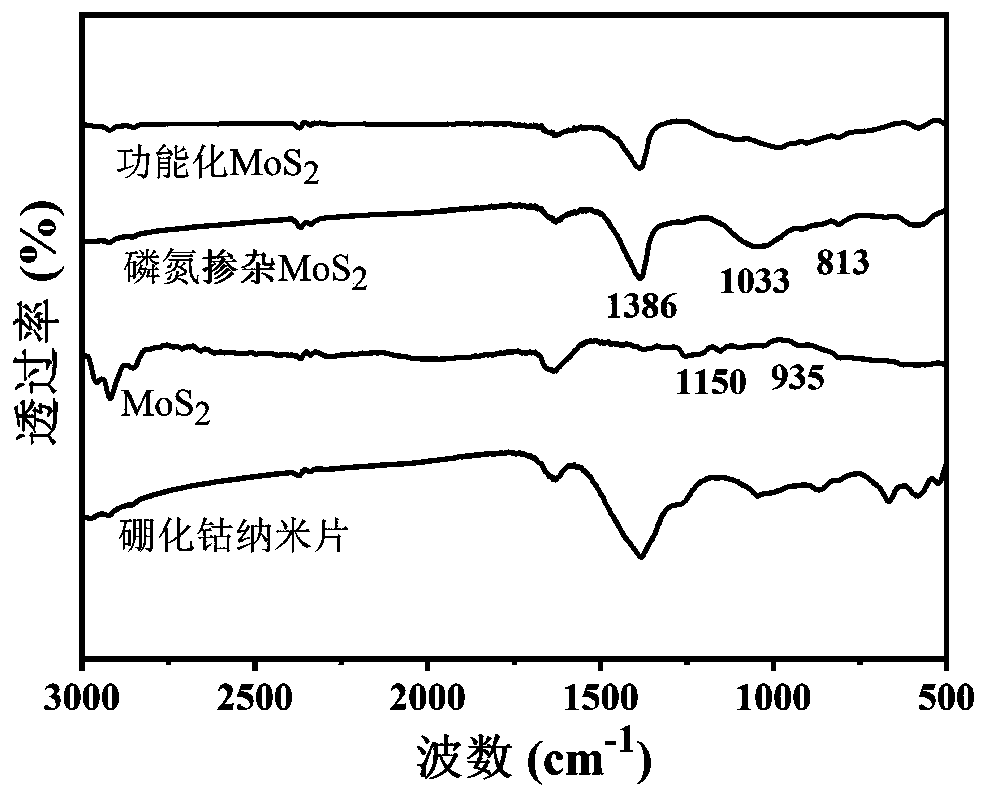
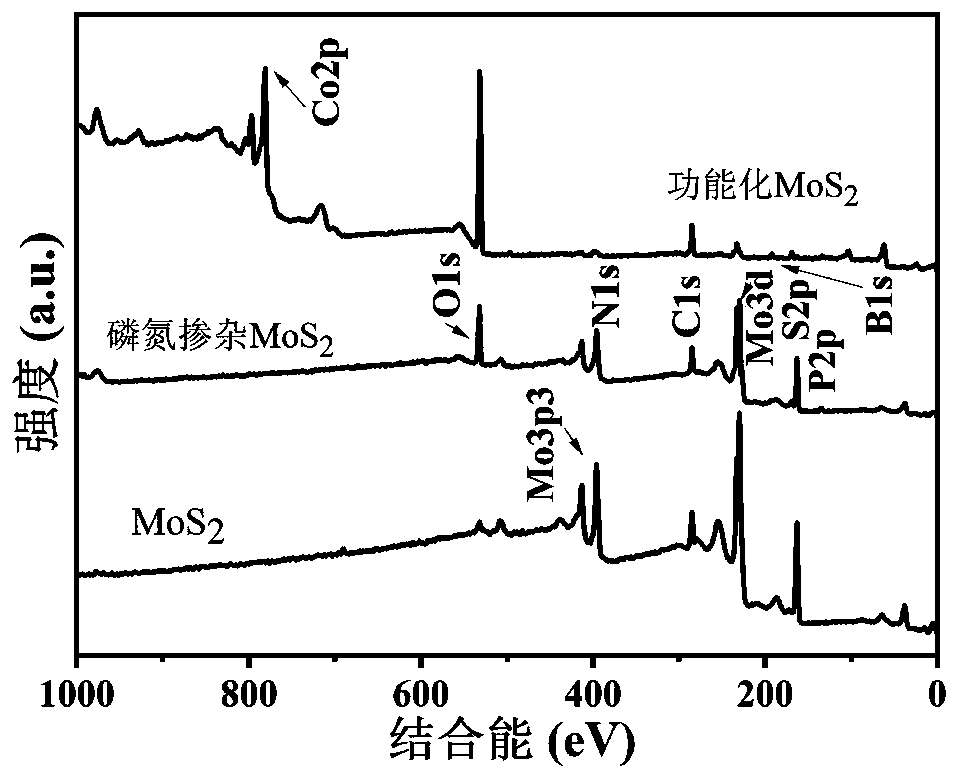
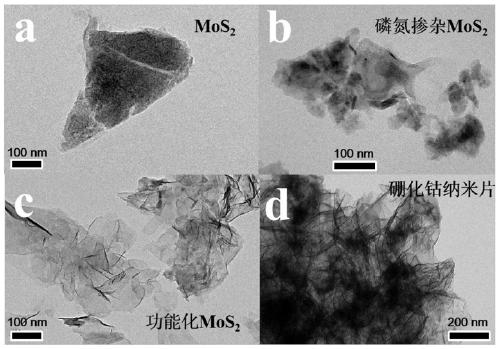
Preparation method of functionalized modified molybdenum disulfide nanosheet
The invention discloses a preparation method of a functionalized modified molybdenum disulfide nanosheet. The preparation method comprises the following steps of: firstly, uniformly mixing phosphonitrilic chloride trimer and guanidine hydrochloride with a given amount of molybdenum disulfide powder through a ball milling method to prepare a molybdenum disulfide nanosheet precursor; putting the precursor into a quartz furnace, preparing an element-doped nanosheet through an annealing process; and finally modifying the surface of a molybdenum disulfide nanosheet with a cobalt boride nanosheet inan in-situ growth manner, and obtaining the functionalized modified molybdenum disulfide nanosheet. The functionalized modified molybdenum disulfide nanosheet provided by the invention is simple in preparation method, low in cost and high in surface modifier content. The prepared modified molybdenum disulfide nanosheet has multifunctionality, for example, the modified molybdenum disulfide nanosheet can be used as a novel solid lubricating material or a hybrid flame retardant with a synergistic effect. In addition, the dispersion state and compatibility of the molybdenum disulfide nanosheet ina polymer matrix is improved, so that the improvement of the comprehensive performance of the polymer material can be benefitted.
Owner:UNIV OF SCI & TECH OF CHINA
Carbon dot-modified carbon nitride/stannic oxide composite photocatalyst, and preparation method and application thereof
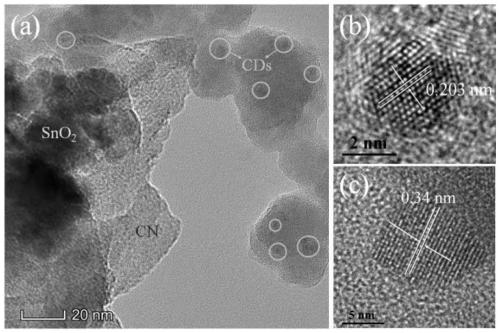
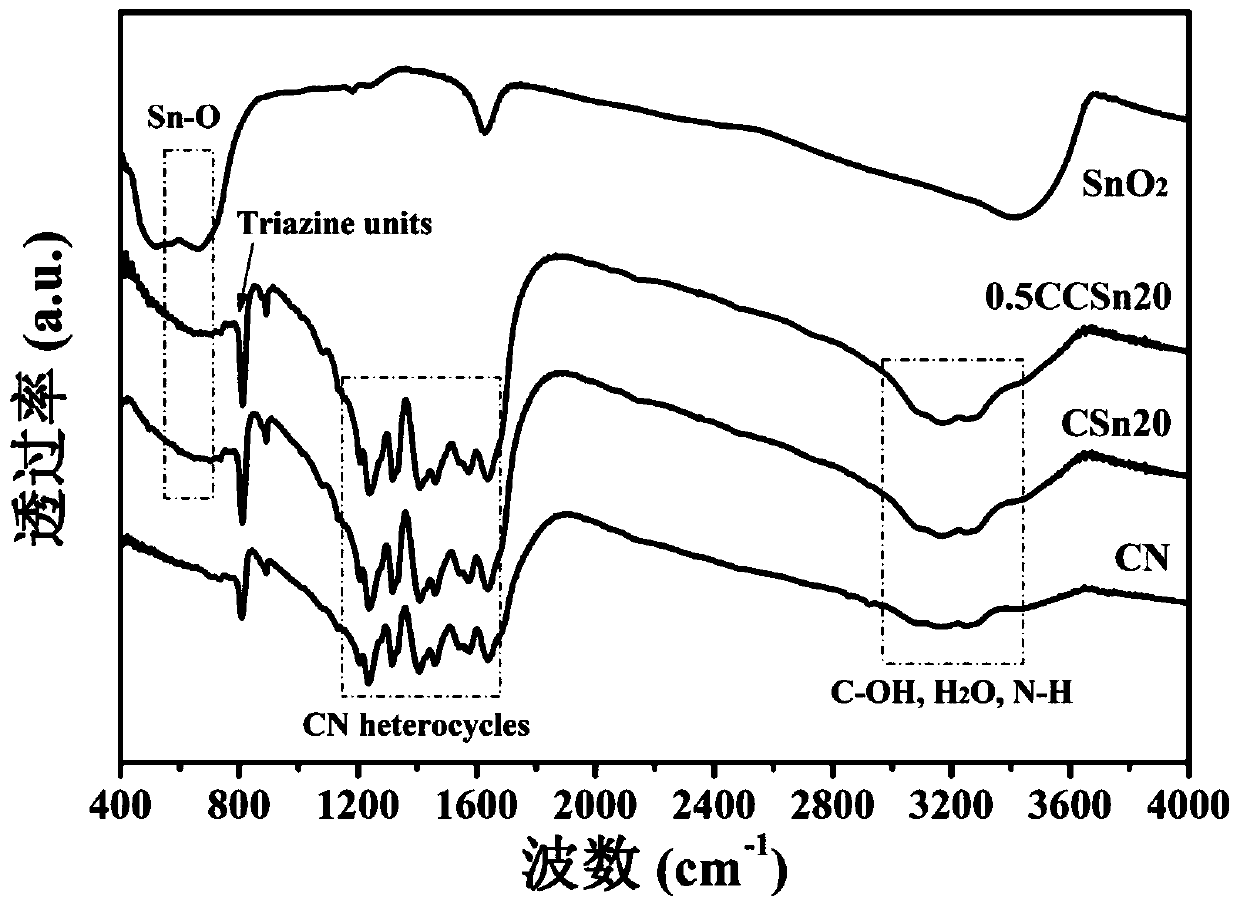
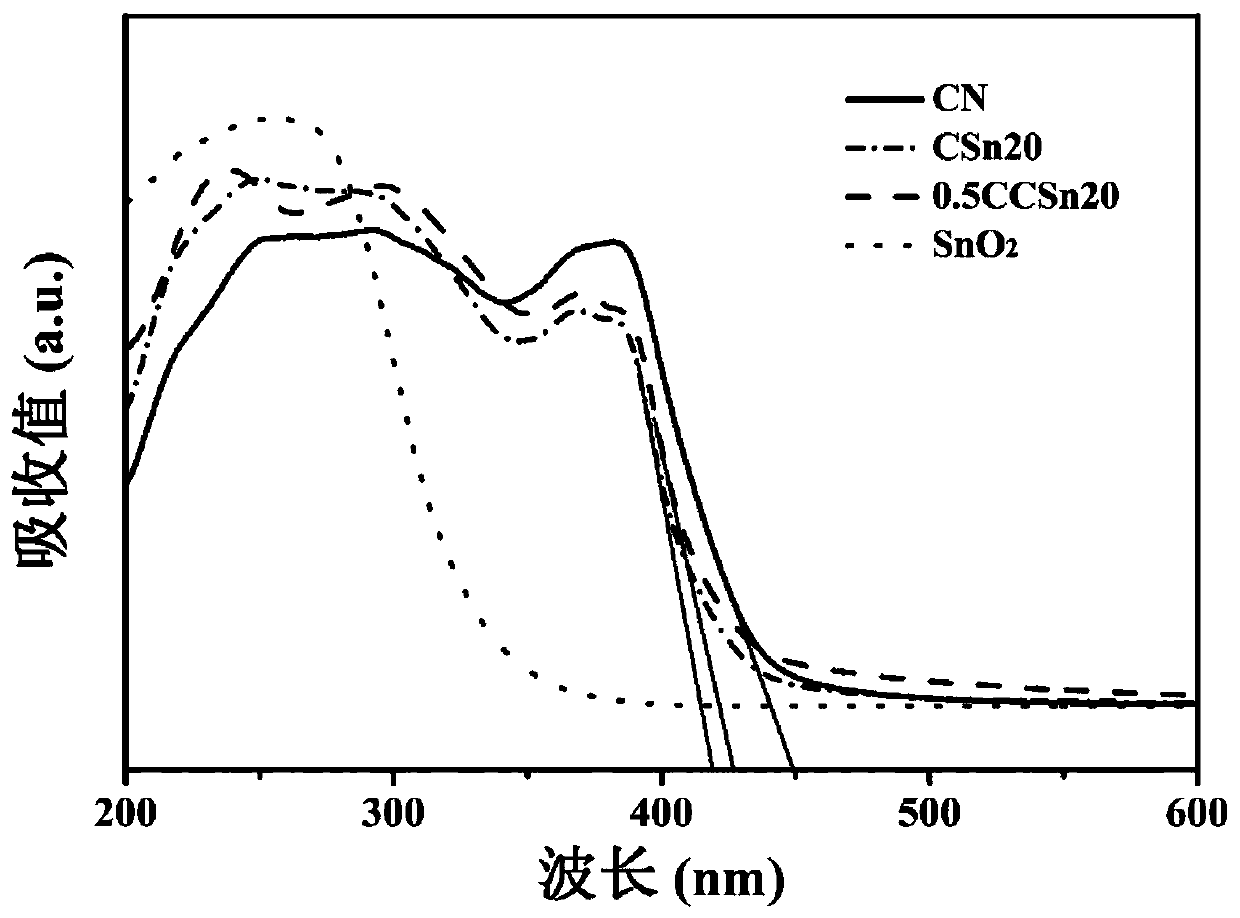
ActiveCN111185215AInhibitory complexImprove photocatalytic efficiencyPhysical/chemical process catalystsWater/sewage treatment by irradiationTin dioxideModified carbon
The invention belongs to the technical field of photocatalytic materials, and discloses preparation and application of a carbon dot-modified carbon nitride / tin dioxide composite photocatalyst. The photocatalyst is prepared through the following steps: calcining guanidine hydrochloride at 500-550 DEG C, and carrying out grinding to obtain carbon nitride powder; dissolving tin tetrachloride pentahydrate in ultrapure water, carrying out heating at 140-160 DEG C, and performing centrifugal cleaning to obtain tin dioxide; dissolving citric acid and urea in ultrapure water, carrying out ultrasonic treatment, conducting heating at 160-180 DEG C, performing centrifuging, collecting a supernatant, and dissolving the supernatant in deionized water to obtain a CDs stock solution; dissolving carbon nitride powder and stannic oxide in ethanol, carrying out heating in a water bath at 60-70 DEG C, calcining the obtained solid at 350-400 DEG C, dissolving the obtained CN / SnO2 and CDs stock solution inethanol, carrying out ultrasonic treatment, performing heating in a water bath at 60-70 DEG C, and calcining the obtained solid at 280-300 DEG C to obtain the photocatalyst. The photocatalyst provided by the invention effectively improves the light absorption performance of a CN / SnO2 photocatalytic material.
Owner:GUANGDONG UNIV OF TECH
Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof
ActiveCN101928277ASimple processLow costOrganic chemistryOrganic compound preparationBenzoic acidMethyl palmoxirate
The invention provides a preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid. The preparation method comprises the following steps of: carrying out a guanidine-forming reaction on 3-amino-4-methyl toluic acid and cyanamide under the acidic condition of hydrochloric acid to generate 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride, and then carrying out a cyclization reaction on the 3-amino-4-methyl toluic acid and cyanamide and 3-(dimethylamino)-1-(3-pyridyl)-2-propylene-1-one to generate the 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, wherein a structural formula of the 3-[(aminoiminomethyl)amino]-4-methyl-benzoic acid-hydrochloride is shown as the description. The method has short route, simple operation, safe and environmentally-friendly process, repeatability, low cost, high yield, high stability and safety of a guanidine hydrochloride intermediate, suitability for large-scale industrial production and higher economic benefit and social benefit.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Nucleic acid extraction kit for excrement sample, preparation method and extraction method
PendingCN114107289ARelease fullyMeet the detection amountMicrobiological testing/measurementDNA preparationPrimary lysisGuanidine
The invention provides a nucleic acid extraction kit for an excrement sample, a preparation method and an extraction method, and relates to the technical field of biology, the kit comprises protease K, a primary lysis buffer solution and a lysis binding buffer solution; the concentration range of the protease K is 10-30 mg / mL; the primary lysis buffer solution comprises Tris-HCl with the pH value of 6.0 to 8.550 to 200 mM, SDS with the concentration of 0.5 to 30 percent (w / v) and EDTA with the concentration of 1 to 10 mM, and the pH value of 8.0 to 9.0; the lysis binding buffer solution is prepared from 50 to 200mM of Tris-HCl, 1 to 15M of guanidine hydrochloride, 1 to 10mM of EDTA (Ethylene Diamine Tetraacetic Acid), 0.1 to 5 percent of TritonX-100 and 0.1 to 5 percent of Tween-20, and the pH (Potential of Hydrogen) of the lysis binding buffer solution is 7.0 to 8.0. The DNA nucleic acid extracted and purified by the kit is high in yield and good in purity, and the problem that the preservation time of the DNA extracted from the excrement sample is short is solved.
Owner:JIANGSU COWIN BIOTECH CO LTD +2
Durable, antistatic and dustproof finishing agent for flocking fabrics and preparation method thereof
The invention discloses a durable, antistatic and dustproof finishing agent for flocking fabrics. The finishing agent is prepared by matching, by weight, 50-100 parts of methyl hydrogen silicone oil, 70-120 parts of allyl polyethoxy polypropoxy ether, 1.5-5 parts of vinyl trialkoxy silane and 0.1-0.16 part of chloroplatinic acid ethanol solution, making the materials subjected to addition reaction to obtain silicone oil co-modified by polyether and alkoxy, adding 480-820 parts of water for dilution and adding 10-20 parts of glyoxal aqueous solution and 400-500 parts of guanidine hydrochloride aqueous solution for mixing. The flocking fabrics finished through the finishing agent are soft and smooth in hand feel and have excellent antistatic properties, and the fabric surface is not prone to adsorb dust.
Owner:LIAONING FIXED STAR FINE CHEM
Method for preparing cartilage angiogenesis inhibiting factor for scalloped hammerhead shark
ActiveCN104894200AProduction suppressor activityAdvanced Extraction ProcessPeptide preparation methodsFermentationVascular endotheliumCell membrane
The invention relates to a method for preparing a cartilage angiogenesis inhibiting factor for a scalloped hammerhead shark. During preparation, Pro-Asp-Tyr-Lys-Phe-Lys is obtained through cartilage homogenate of the scalloped hammerhead shark, guanidine hydrochloride extraction, acetone precipitation and grading, enzymolysis, gel filtration chromatography, cell membrane chromatography purification and efficient liquid phase chromatography purification. The angiogenesis inhibiting factor can effectively inhibit angiogenesis of chick chorioallantoic membranes, and has an obvious inhibition function on expression of three angiogenesis promoting factors including the vascular endothelial cell growth factor, the basic fibroblast growth factor and the platelet-derived growth factor of lung cancer tissue of mice suffering from the Lewis lung cancer.
Owner:ZHEJIANG OCEAN UNIV
Wood floor adhesive
InactiveCN104293250AImprove adhesionNot easy to fall offNon-macromolecular adhesive additivesAdhesive cementSodium acetate
The invention discloses a wood floor adhesive prepared from the following raw materials in parts by weight: 5-10 parts of phenylalanine, 0.5-1.7 parts of titanium dioxide, 1.5-3 parts of trihydroxytriethylamine, 2.5-4.3 parts of fatty alcohol alkanolamide, 1.5-2.8 parts of steam explosion lignin, 1.2-3.6 parts of polyethylidene maleate, 0.5-1.2 parts of sodium diacetate and 0.9-2.2 parts of guanidine hydrochloride. Compared with the existing adhesive, the wood floor adhesive disclosed by the invention is strong in adhesive force, not easy to fall off, available in raw materials, simple in preparation process, benzene-free, formaldehyde-free, nontoxic, innocuous, environment-friendly and low-carbon and also has the advantages of high solid content, small shrinkage rate, good initial adhesiveness, high drying speed, freeze prevention, simplicity in gluing and the like.
Owner:王璐
Nano multidirectional chromatography nucleic acid extraction medium and preparation method thereof
ActiveCN106607013APreserve integrityAvoid degradationOther chemical processesDNA preparationMacromoleculeHydrochloride
The invention discloses a nano multidirectional chromatography nucleic acid extraction medium, which has a substrate of a macromolecular carbohydrate and an extract penetrating the substrate. The extract comprises a mixture of guanidine hydrochloride, SDS and EDTA located at a first layer, a protective agent located at a second layer, a mixture of guanidine hydrochloride, SDS and EDTA located at a third layer, and a protective agent located at a fourth layer. And the mixture of guanidine hydrochloride, SDS and EDTA located at the first layer and the mixture of guanidine hydrochloride, SDS and EDTA located at the third layer are different in content. The substrate can well preserve the nucleic acid integrity and prevent nucleic acid degradation.
Owner:SUZHOU HAIMIAO BIOTECH CO LTD
Nucleic acid purifying reagent and method
ActiveCN111235145AImprove purification efficiencyHigh purityDNA preparationSodium acetateMonosodium glutamate
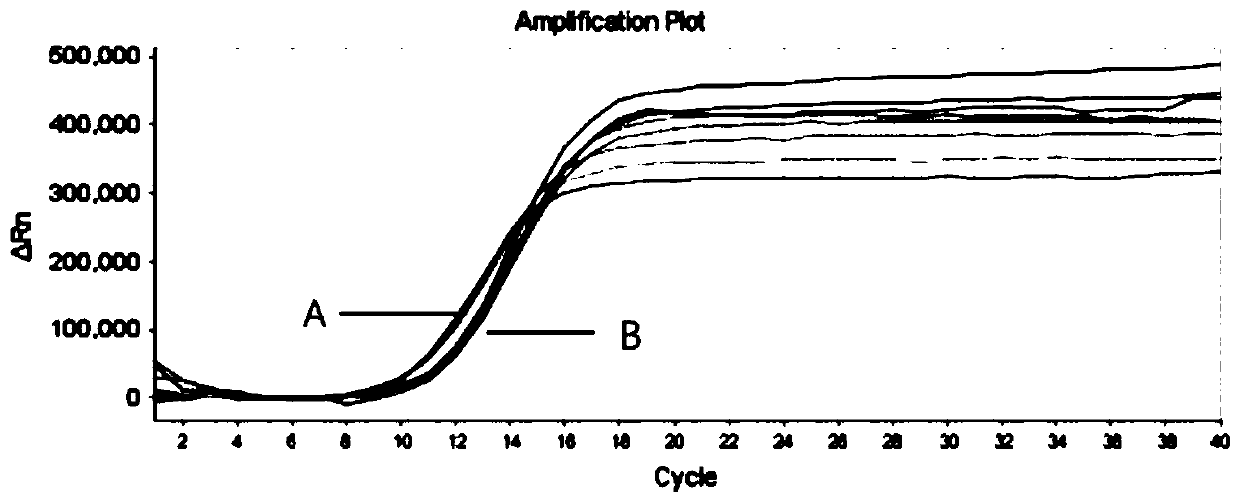
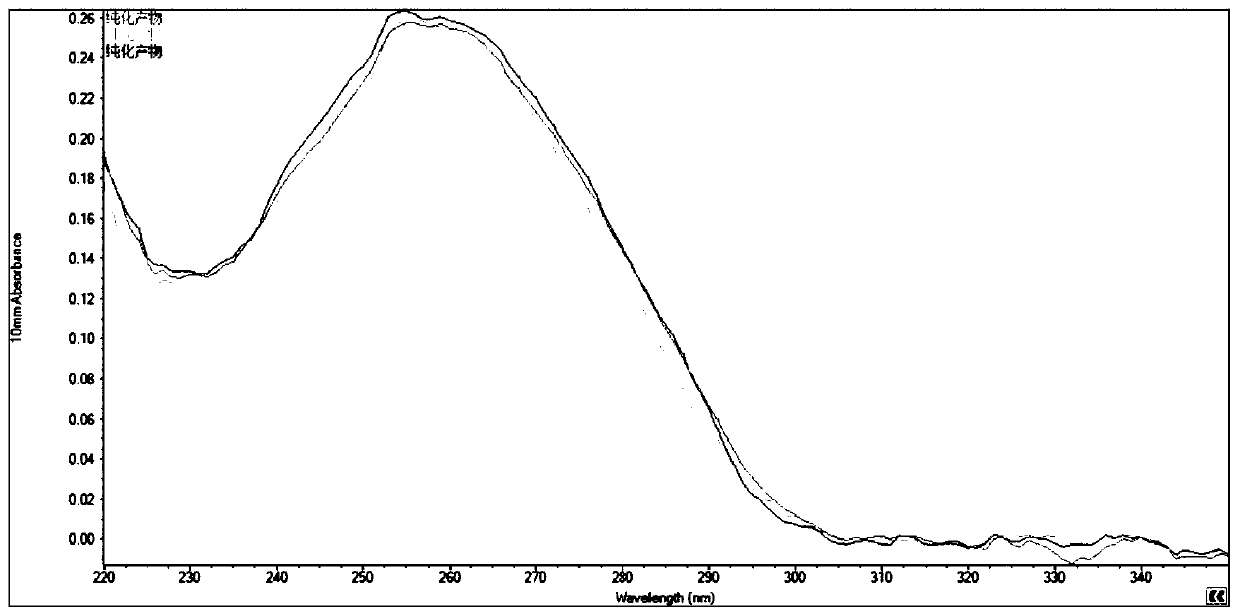
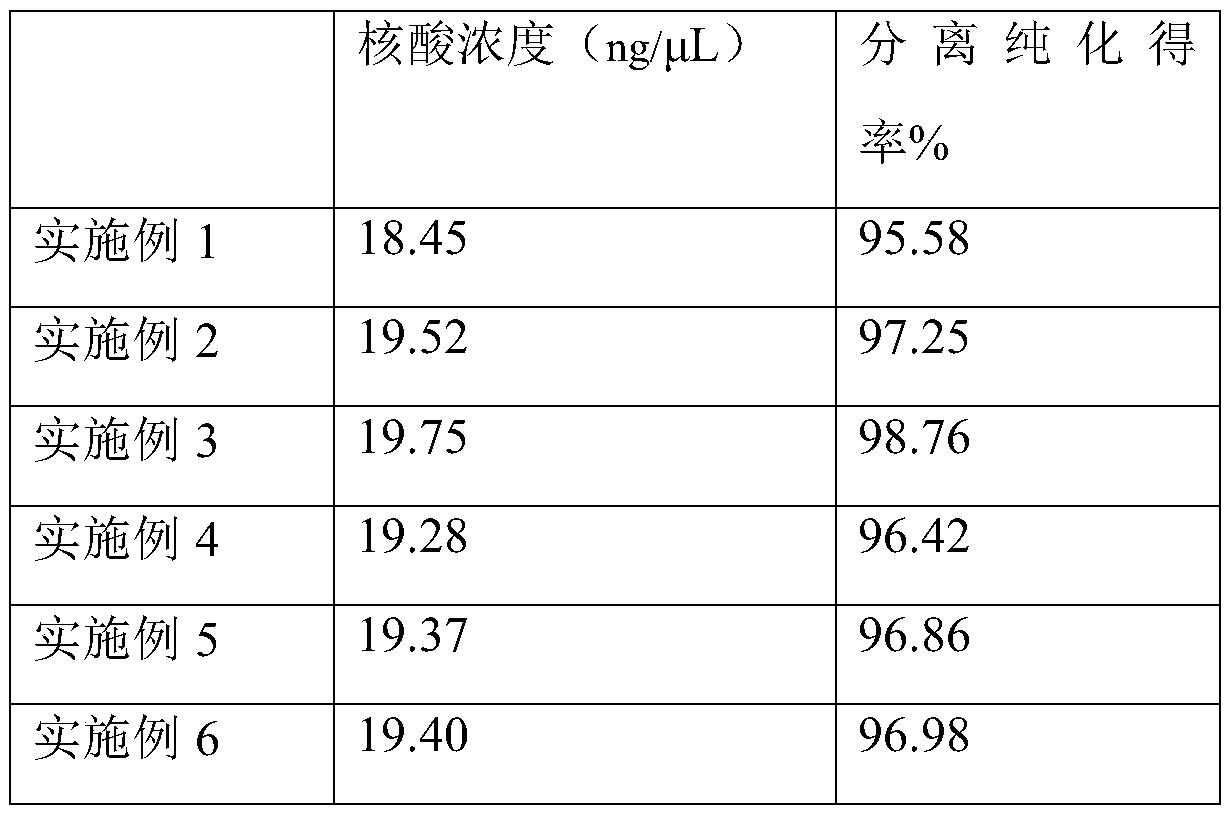
The invention discloses a nucleic acid purifying reagent and method, and belongs to the biological field. The reagent contains magnetic beads, DNA binding liquid, a scrubbing solution and eluant, wherein the magnetic beads are silicon-hydroxyl-modified magnetic beads; the DNA binding liquid is guanidine hydrochloride, sodium acetate and Triton X-100; the scrubbing solution includes a reagent 1 including guanidine hydrochloride, sodium acetate and Triton X-100 as well as a reagent 2 including ethanol, sodium acetate and sodium citrate; and the eluant is Tris-HCl. By adding sodium cocoyl glutamate and sodium lauryl glutamate into the DNA binding liquid in a concentration ratio of 1 to (1-5) in an implementation process, a purification effect can be obviously improved. By virtue of interaction of the reagents, the purification efficiency of nucleic acid is obviously improved, the purity is high, and the separation purification yield can reach up to 98.76% and is obviously higher than thatin the prior art.
Owner:INTEGRATED BIOSYSTEMS CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
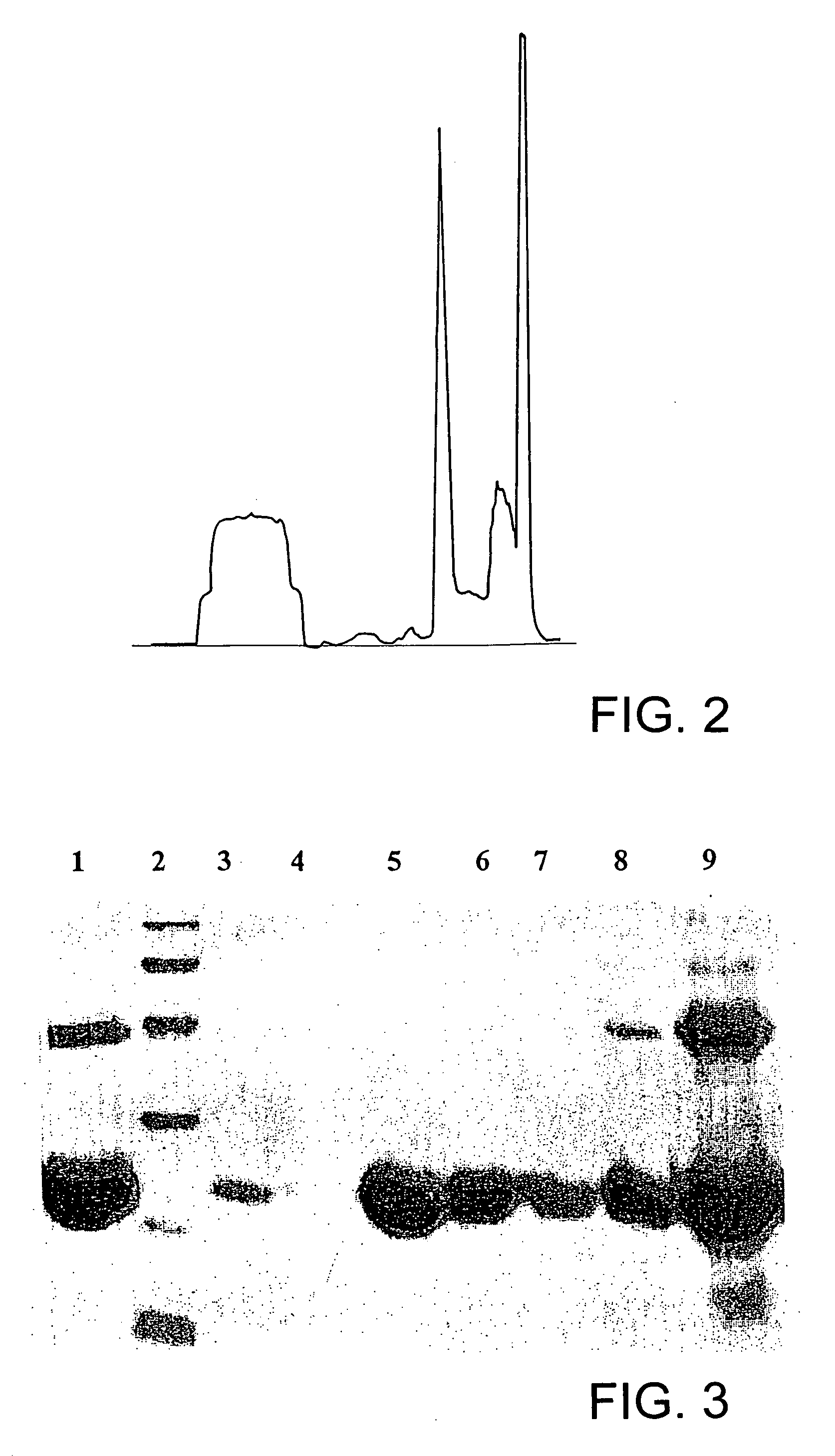
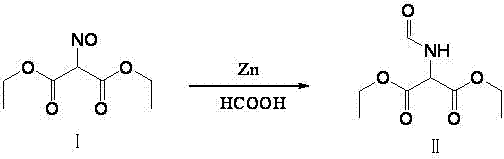
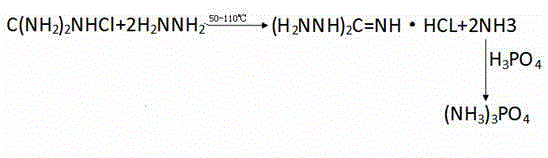
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka.patsnap.com/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000001.png)
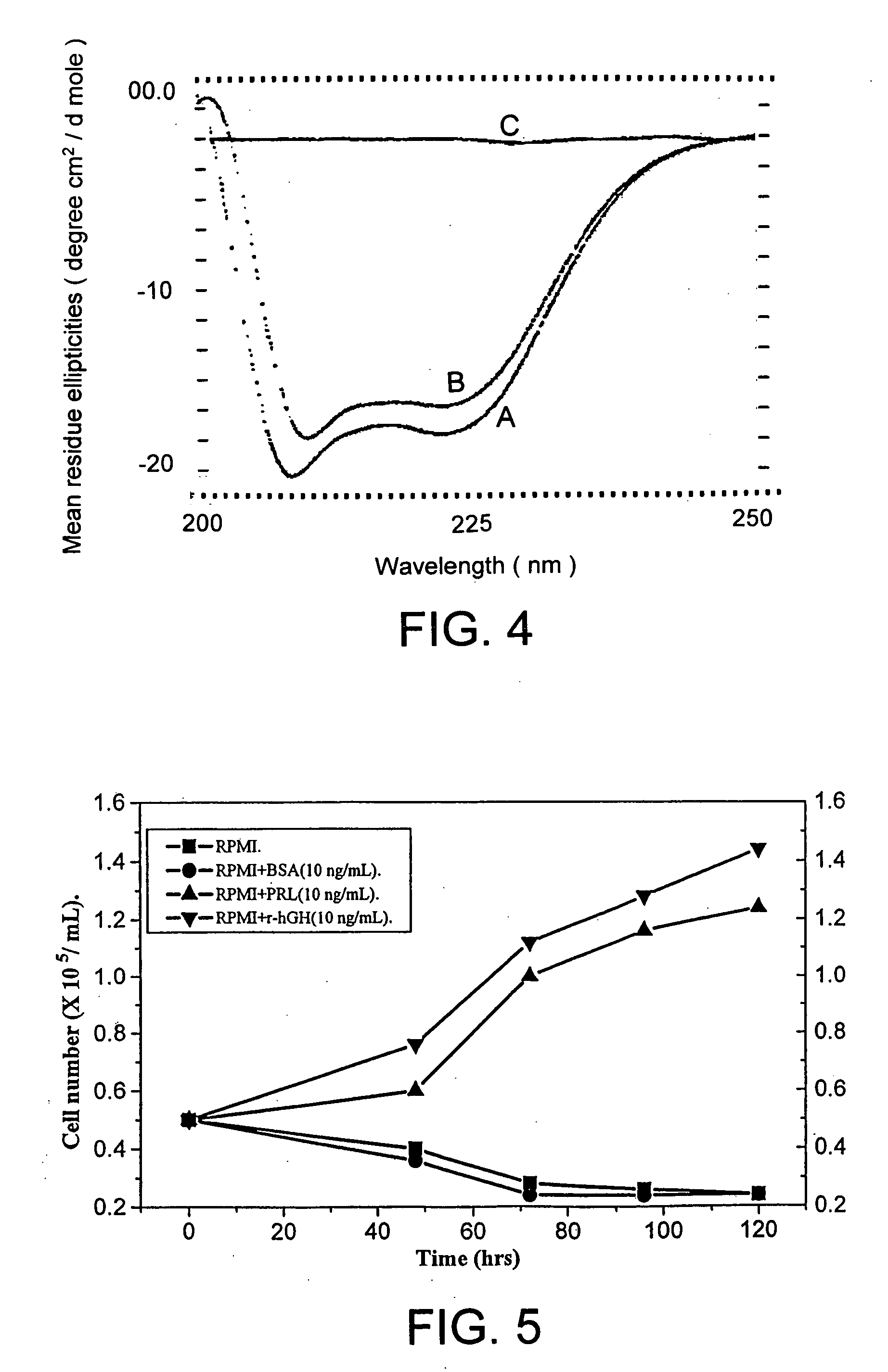
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka.patsnap.com/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000002.png)
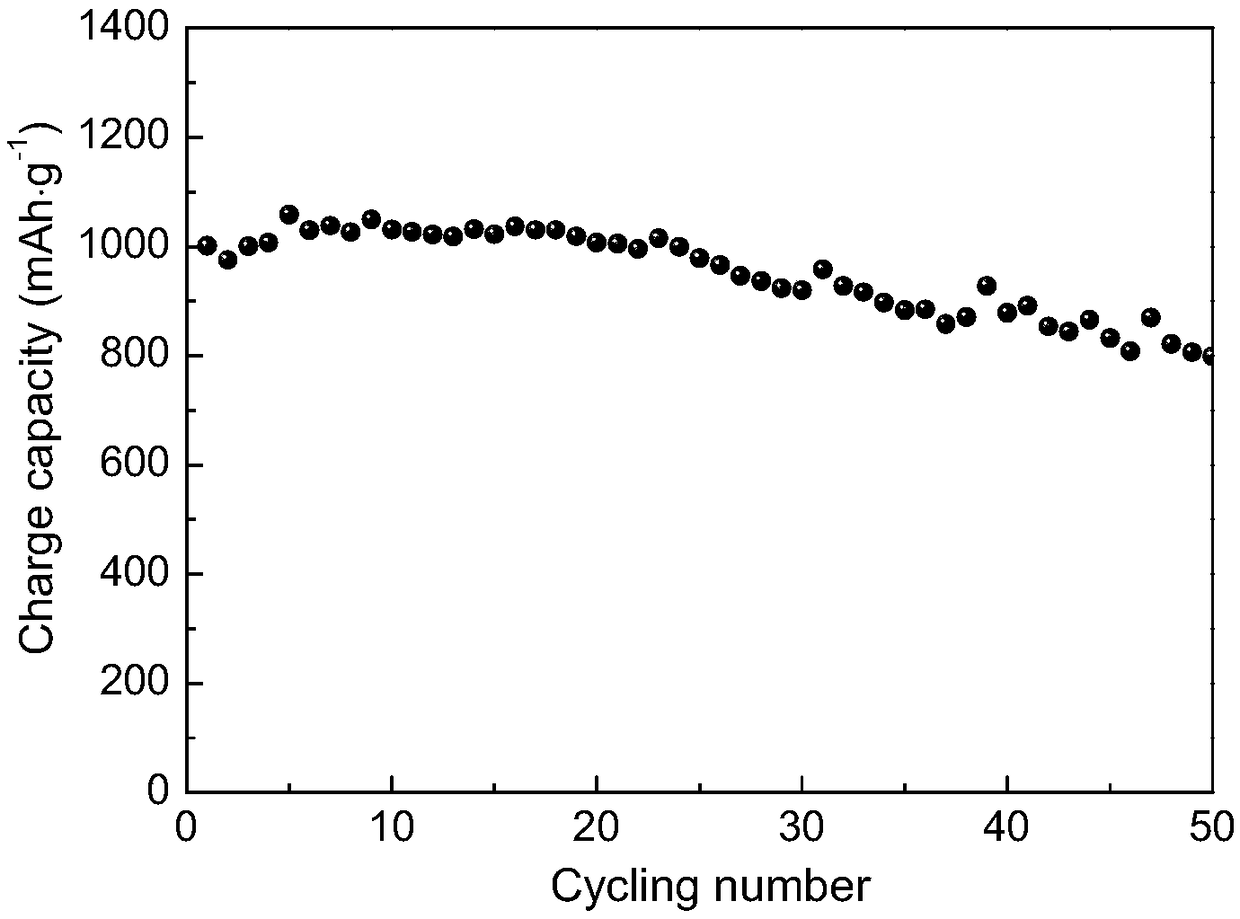
![Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof Preparation method of 4-methyl-3-[[4-(3-pyridyl)-2-pyrimidyl]amino]benzoic acid, related intermediate and application thereof](https://images-eureka.patsnap.com/patent_img/a80a22c8-4078-4726-ba46-b0c4d0b99c59/000003.png)