Kit for extracting blood free DNA
A kit and blood technology, applied in the field of kits for the extraction of free DNA from blood, can solve the problems of low purity and low recovery efficiency, and achieve the effect of improving extraction efficiency, improving recovery efficiency, and reducing material costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
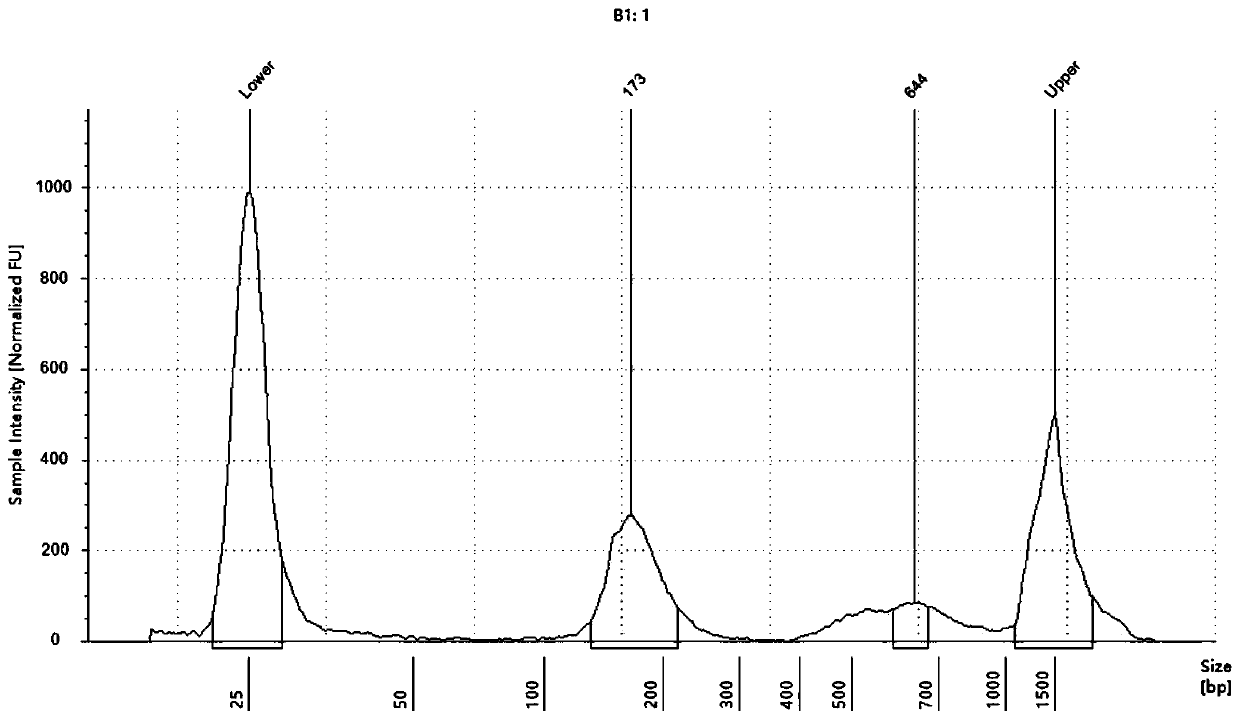
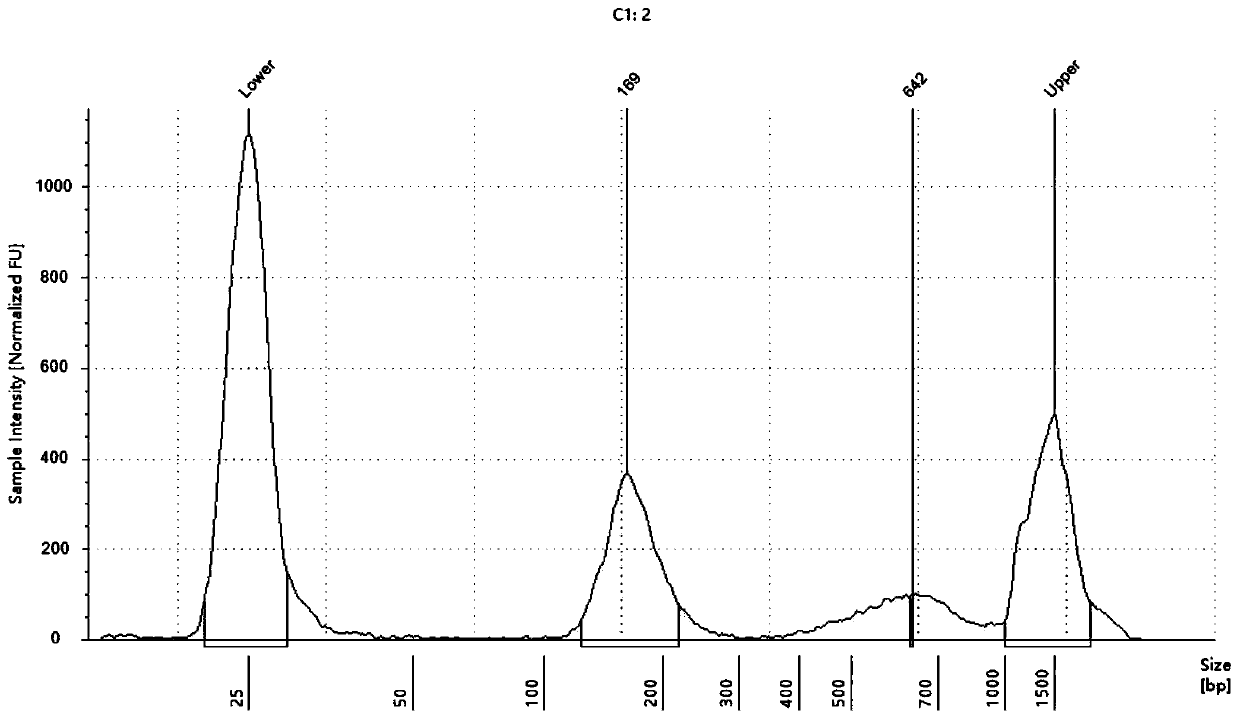
[0042] The kit in this example specifically includes lysate, proteinase K, magnetic bead suspension, binding solution, cleaning solution 1, cleaning solution 2 and eluent.
[0043] Wherein, the lysate is composed of 4M guanidine isothiocyanate, 50mM Tris-HCl, 25mM EDTA, 300mM NaCl and 12% Triton X-100.
[0044] Proteinase K was purchased from Tiangen Biochemical Technology, and the magnetic bead suspension was purchased from Weidu Biotechnology.
[0045] The binding solution consisted of 3.5M guanidine isothiocyanate, 50 mM Tris-HCl, 25 mM EDTA, 12% Triton X-100 and 30% isopropanol.
[0046] Cleaning solution 1 consisted of 2M guanidine isothiocyanate, 50 mM Tris-HCl, 25 mM EDTA, 5% TritonX-100 and 30% isopropanol.
[0047] Wash solution 2 consisted of 10 mM Tris-HCl and 80% absolute ethanol.
[0048] The eluent was nuclease-free sterile deionized water.
[0049] The method of using the kit in this example is as follows:
[0050] (1) Add 40 μL of proteinase K, 1 mL of plas...
Embodiment 2
[0064] In this example, on the basis of Example 1, the components and concentrations of the components of the lysate, the binding solution, the cleaning solution 1 and the cleaning solution 2 were tested, as follows in detail:
[0065] 1. Lysate formulation test
[0066] In this example, different concentrations of the components in the lysate were tested, as shown in Table 2.
[0067] Table 2 Lysate formula test
[0068]
[0069]
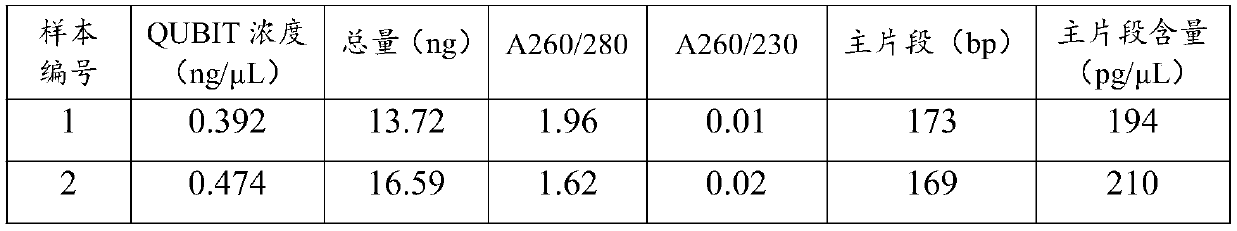
[0070] In this example, according to Table 2, the lysates of eight formulations from Test 1 to Test 8 were prepared, and the rest of the reagents, such as proteinase K, binding solution, cleaning solution 1 and cleaning solution 2, etc., were the same as in Example 1, and the same as in Example 1. According to the method, cfDNA was extracted from the plasma sample 1 of Example 1. And measure the concentration and purity of the extracted cfDNA according to the method of Example 1. The results show that the lysates of the eight formulations ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com