4'-pyridylpyrimidine compound and synthetic method and application thereof
A kind of technology of pyridyl pyrimidine and synthesis method, which is applied in the field of fine organic synthesis and achieves the effect of good practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic method of 4'-pyridyl pyrimidine compound, synthetic process is:
[0030]
[0031] Specific steps are as follows:
[0032] 1) Preparation of 7-(pyridin-4'-yl-methylene) isolongifanone:
[0033] Put 8mmol of isolongifolanone, 12mmol of 4-pyridinecarbaldehyde, 16mmol of sodium ethoxide and 30mL of ethanol in a three-necked flask equipped with a stirrer, a thermometer and a reflux condenser in sequence, and heat it to reflux at 80-90°C to react for 5 hours From about to isolonganone, the conversion rate reaches over 95% (GC tracking detection). The reactant was extracted 3 times with 25 mL of ethyl acetate, the organic phases were combined, and then washed several times with saturated brine until neutral, and the organic phase was dried with anhydrous sodium sulfate; the solvent was recovered by filtration and concentration to obtain 7-(pyridine-4 '-yl-methylene) isolongifolanone; carry out recrystallization with ethanol-ethyl acetate, obtain colorless an...
Embodiment 2
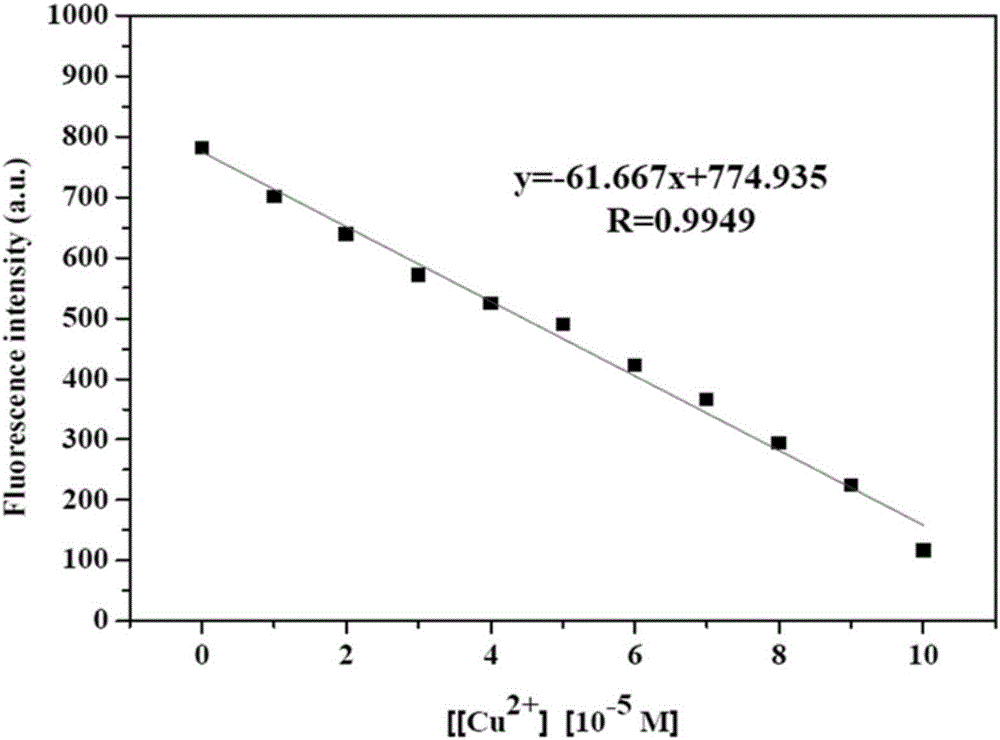
[0037] The 6,6,10,10-tetramethyl-4-(pyridin-4'-yl)-5,7,8,9,10,10a-hexahydro-6H-6a,9- Endomethylenebenzo-2-quinazolinamine formulated as 1 × 10 -5 M's HEPES buffer solution (20mM, pH=7.2, 80% (v / v) C 2 h 5 OH), the Cu 2+ Ions were formulated with the same HEPES buffer at a concentration of 0.1 to 1.0×10 -4 M's solution. Measured different concentrations of Cu 2+ Ion pair 6,6,10,10-tetramethyl-4-(pyridin-4′-yl)-5,7,8,9,10,10a-hexahydro-6H-6a,9-methanomethene The fluorescence intensity of benzo-2-quinazolinamine, then carry out linear fitting to the fluorescence intensity at 450nm place, obtain as figure 1 The straight line shown, further calculated by the formula, the detection limit of the compound for copper ions is as low as 4×10 -8 M.
Embodiment 3
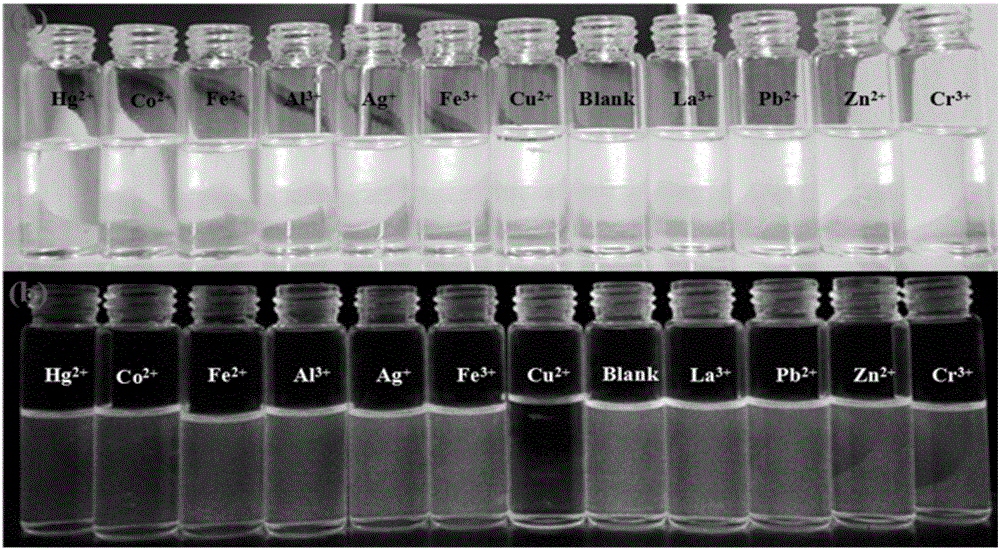
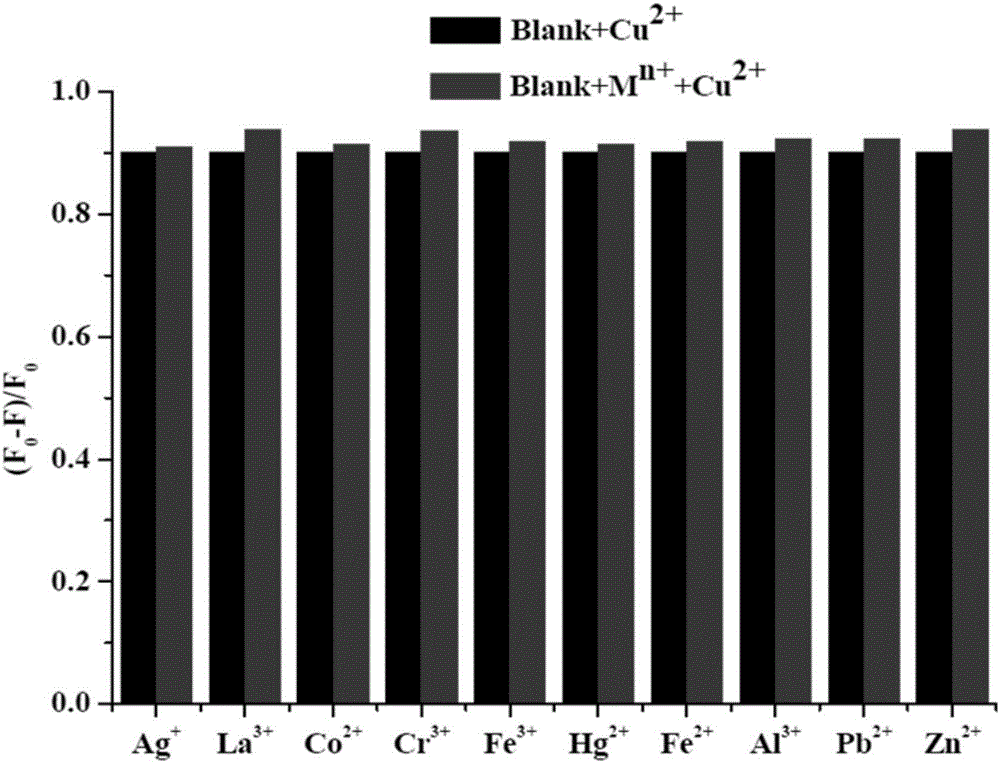
[0039] 6,6,10,10-tetramethyl-4-(pyridin-4′-yl)-5,7,8,9,10,10a-hexahydro-6H-6a,9-methanobenzene And-2-quinazolinamine was dissolved in pure ethanol to prepare a concentration of 1×10 -5 The solution of M, its solution is colorless and transparent under fluorescent lamp, with 1.5×10 -4 The addition of different metal ions of M, the color of the solution did not change significantly, and then observed under a 365nm ultraviolet lamp, as figure 2 As shown, adding Cu 2+ The blue fluorescence of the solution of ions has disappeared, and by adding the same amount of other metal ions, such as Hg 2+ ,Al 3+ , Ag + ,La 3+ ,Pb 2+ , Fe 3+ , Fe 2+ ,Cr 3+ ,Co 2+ ,Zn 2+ Plasma contrast, the fluorescence of the compound did not change significantly, indicating that the compound can be used as an effective identification of Cu 2+ Ionic fluorescent probes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com