Visualization interactive clinical test and clinical follow-up visit system and method
A clinical trial, interactive technology, applied in the field of clinical information system, can solve the problems of lack of clinical trial or clinical follow-up data auxiliary input function, inability to realize the input monitoring feedback loop process, waste of human, financial and material resources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
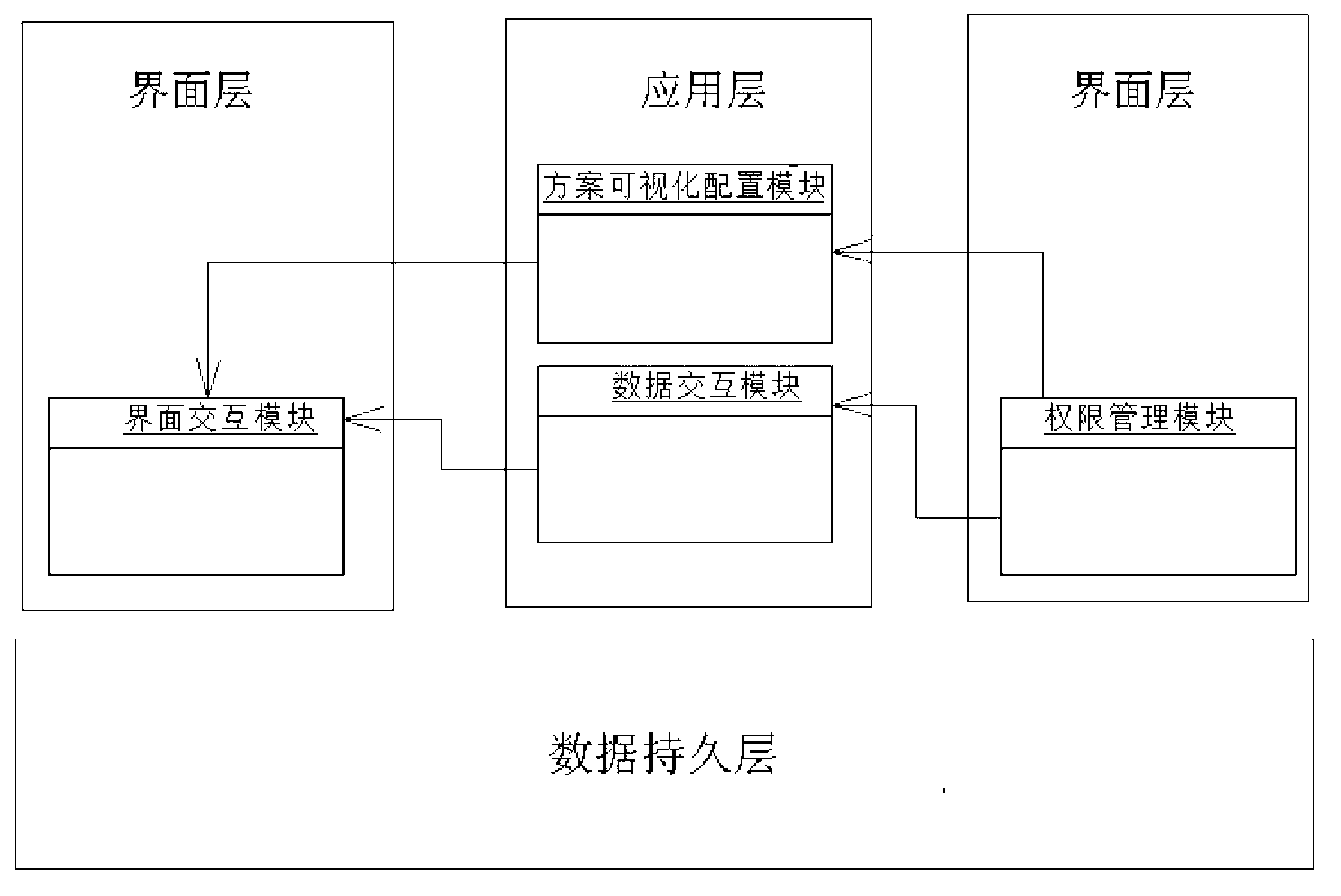
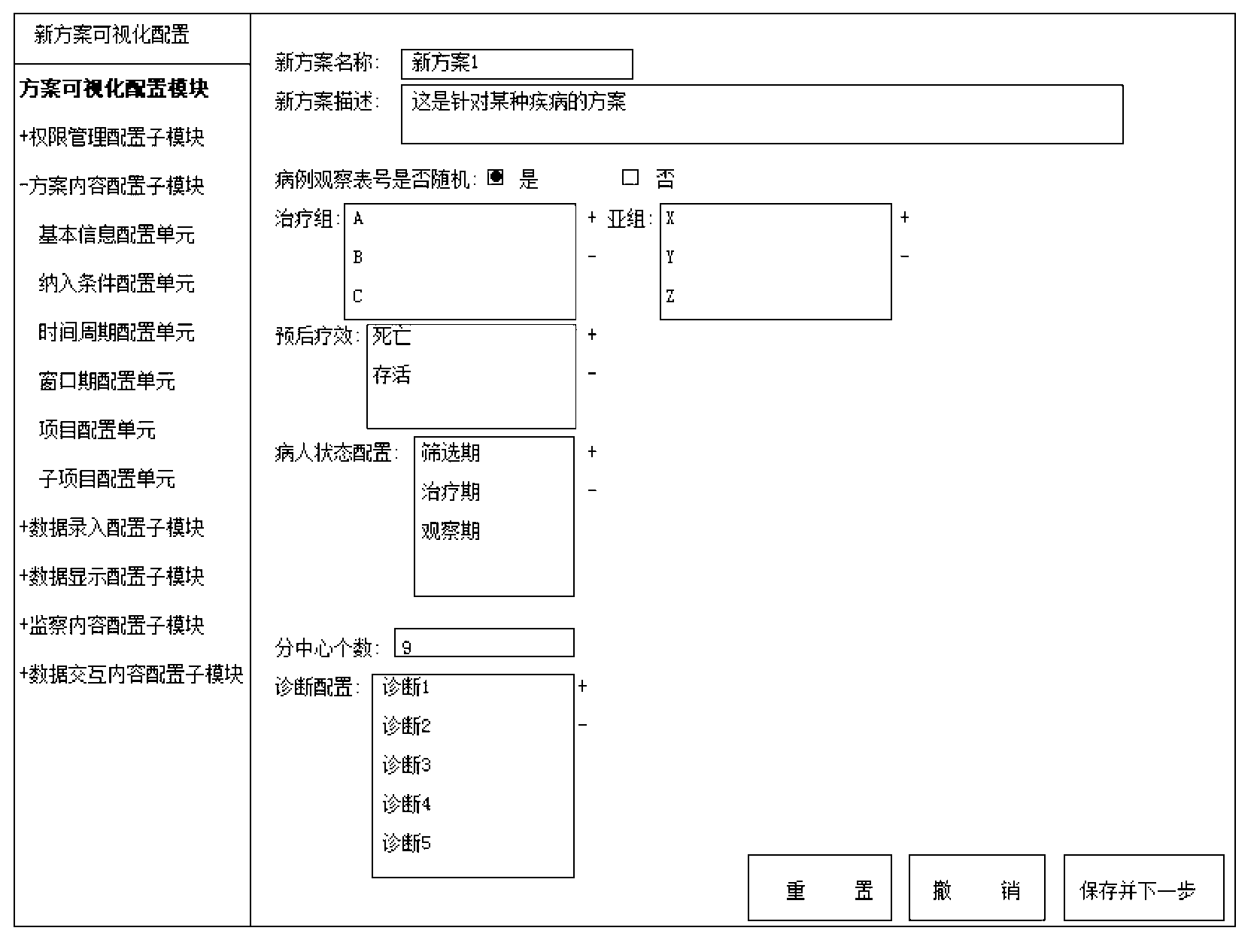
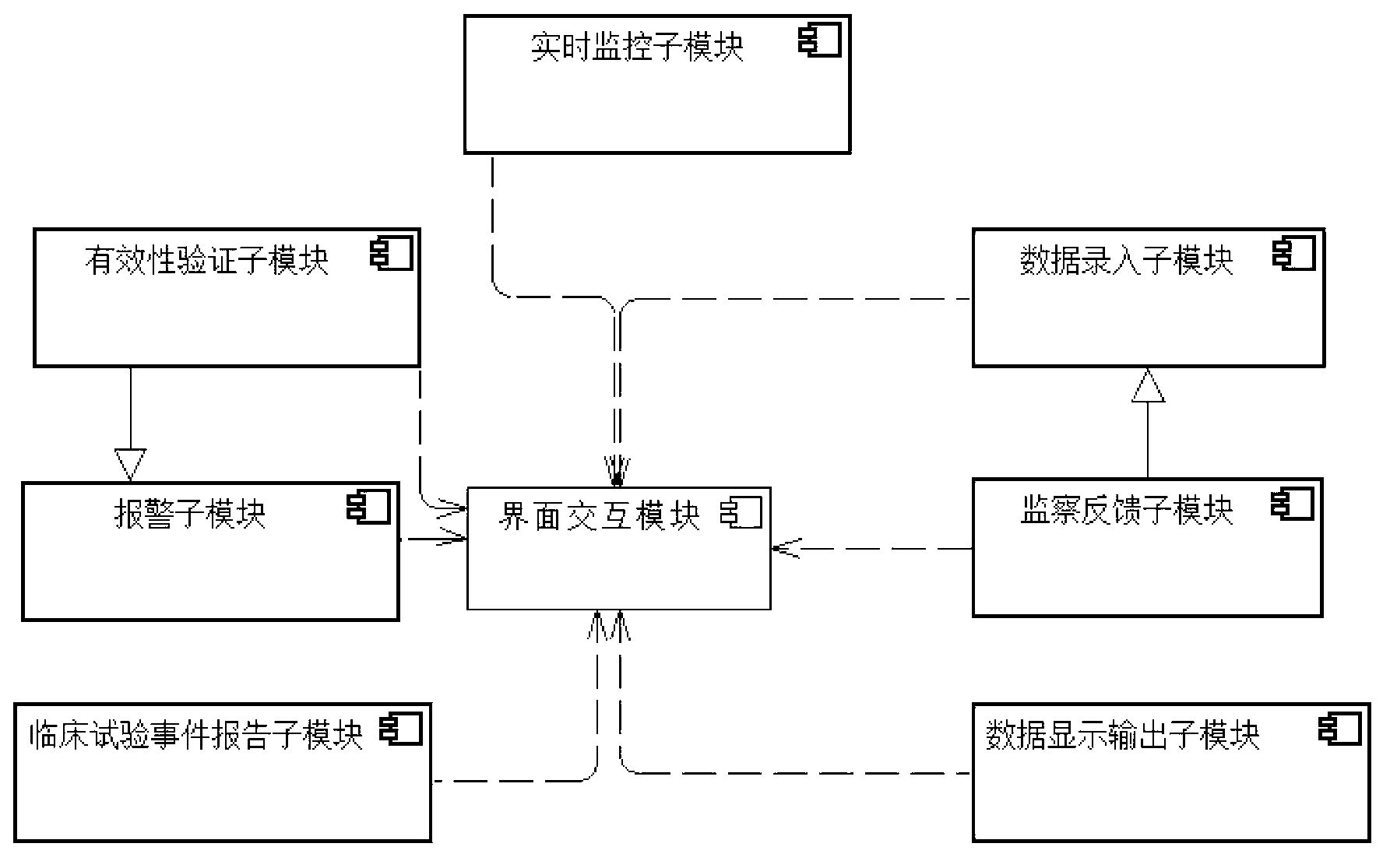
[0102]The user intends to conduct a multi-center, prospective, randomized controlled clinical trial of a certain drug. Firstly, according to the requirements of the clinical trial and clinical follow-up plan, the user sets the identity of the system administrator, the administrator of the leading unit, the general entry of each center, the entry of the director of each center, the supervisor and other user identities and their corresponding data entry in the authority management module , modify, delete, export, print, statistical analysis and monitoring permissions. Secondly, the user takes the established plan as the blueprint, and in the plan visualization configuration module, configures the content of various interface interaction modules and data interaction modules (such as visit point, window period, project name of each visit point, sub-items contained in each project, each Valid value verification rules of sub-items, header combination of patient list, etc.) to comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com