Beta-carboline ruthenium compound as well as preparation method and application thereof
A complex, ruthenium carboline technology, applied in the field of β-carboline ruthenium complexes and their preparation of autophagy inducers as anti-tumor drugs, can solve the problems of autophagy-inducing gene Beclin1 loss, loss of tumor inhibitory effect, etc., to achieve Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
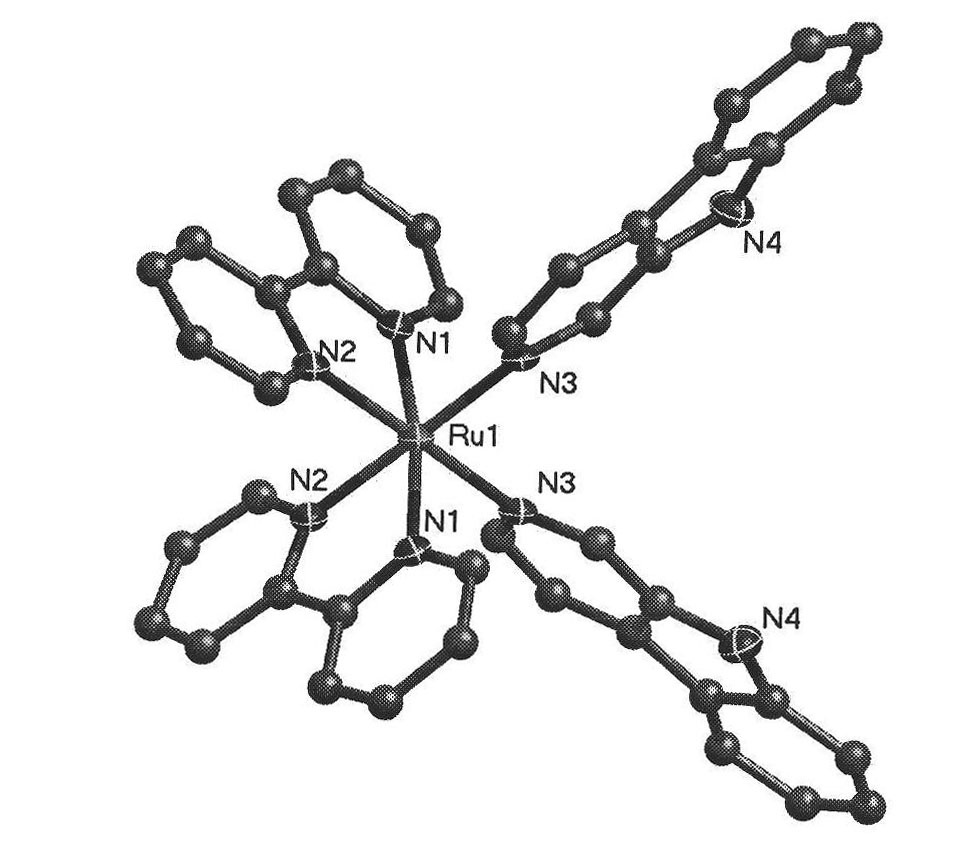
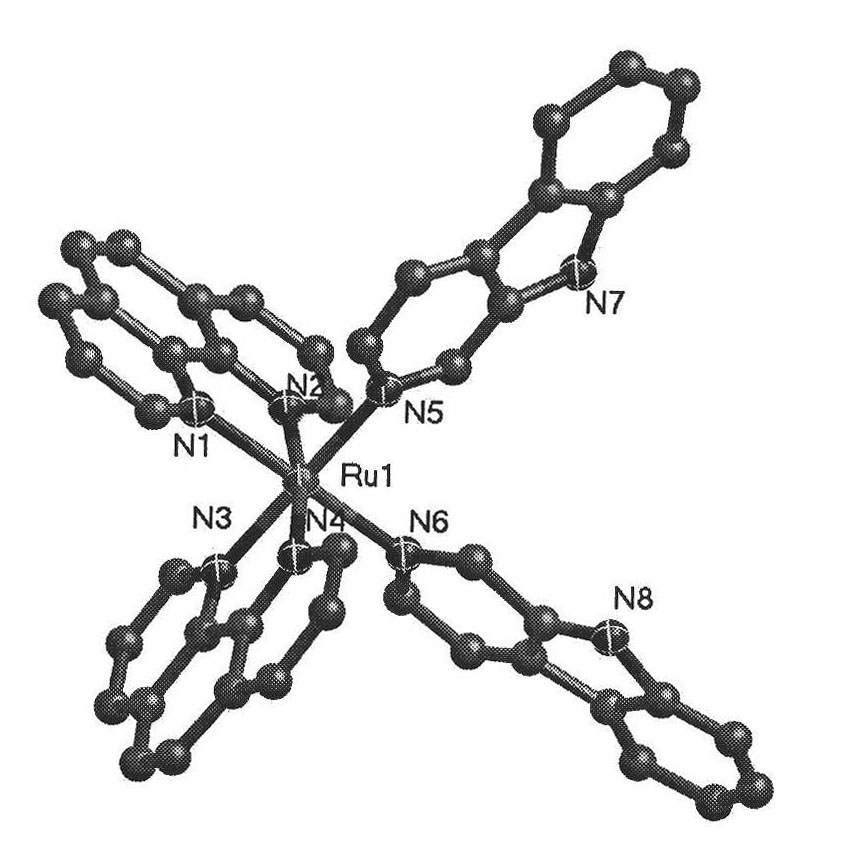
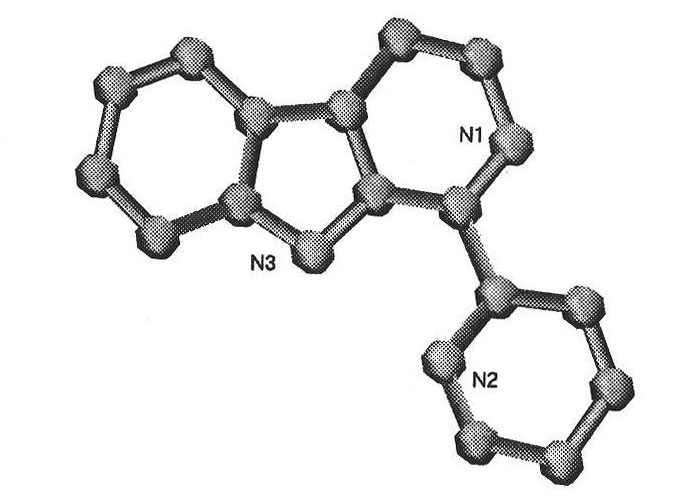
[0025] The β-carboline ruthenium complex of the present invention has a molecular formula of [Ru(N^N) 2 (Nh) X Cl Y ](A^A) z , wherein N^N is selected from bpy or phen; A^A is selected from PF 6 or SO 3 CF 3 ; X=1 or 2, Y=2-X, Z=1 or 2; The molecular structural formula of the cationic moiety corresponding to the complex is shown in A1, A2, A4, A5:
[0026]
[0027] The preparation method of above-mentioned complex, will cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O or cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O and 9H-pyridino[3,4-b]indole (Norharman) were dissolved in aqueous ethanol, refluxed, evaporated under reduced pressure, filtered to remove unreacted 9H-pyridino[3,4-b]indole ligand, added NH 4 PF 6 , after the product is separated out, it is washed and then vacuum-dried, purified and recrystallized to obtain [Ru(bpy) 2 (Nh)Cl](PF 6 ) or [Ru(phen) 2 (Nh)Cl](PF6 ); or, put cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O or cis-[Ru(bpy) 2 Cl 2 ]·2H 2 O and AgSO 3 CF 3 After the reaction, AgCl was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com