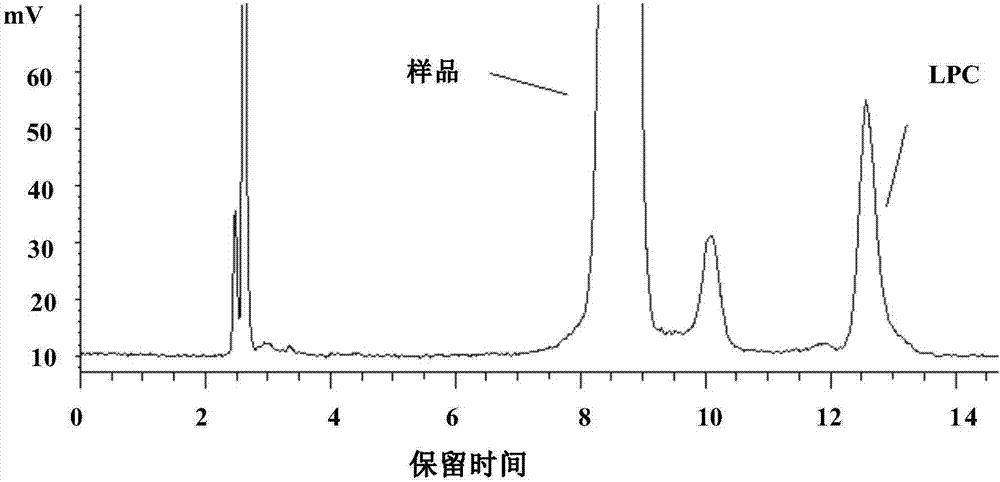
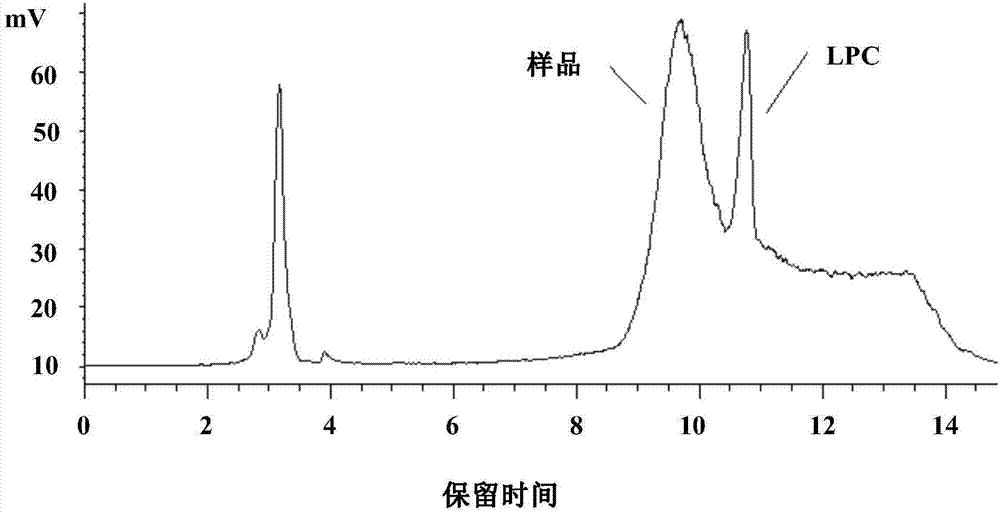
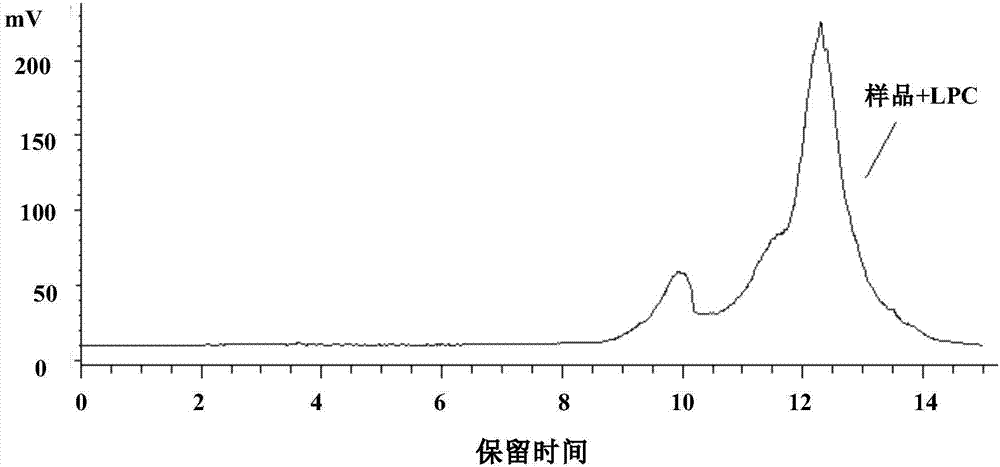
Method for separating and measuring lysophosphatidylcholine in drug preparations
A technology for phosphatidylcholine and pharmaceutical preparations, applied in the field of drug analysis, can solve the problems of inability to achieve separation of lysophosphatidylcholine, influence of HPLC column performance, inability to separate samples of injections, etc., so as to ensure drug quality and good separation. , the effect of the simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Separation and determination of lysophosphatidylcholine in injection by liquid chromatography, the steps are as follows:
[0033] 1. Blank solution: diluent
[0034] 2. Preparation of blank excipient solution:
[0035] Take 1mL of blank injection solution (without egg yolk lecithin), put it in a 10mL measuring bottle, add diluent to dilute to the mark, shake well, and use it as the blank sample solution.
[0036] 3. Preparation of system suitability solution:
[0037] Weigh an appropriate amount of egg yolk lecithin and lysophosphatidylcholine standard substance, add methanol to dilute into a mixed solution containing about 1.2mg of phosphatidylcholine and 0.09mg of lysophosphatidylcholine per 1mL, as the system adaptability of lysophosphatidylcholine solution.
[0038] 4. Preparation of reference solution:
[0039] Accurately weigh an appropriate amount of lysophosphatidylcholine reference substance, add diluent to dissolve to make a solution containing 0.9mg per 1...
Embodiment 2
[0051] Example 2 Recovery test
[0052] Preparation of recovery solution:
[0053] Recovery rate solution: Accurately measure 1ml of alprostadil injection sample, put it in a 10ml measuring bottle, add lysophosphatidylcholine solution equivalent to 80%, 100%, 120% of the limit, add diluent to dilute to the scale, shake well , as the recovery solution. Three replicates were prepared for each concentration.
[0054] Accurately measure 20 μL of the three different concentrations of recovery solutions prepared above, inject them into a liquid chromatograph, use the mobile phase and chromatographic conditions in Example 1, and record the chromatograms.
[0055] According to the measured peak area, the recovery rate of lysophosphatidylcholine was calculated, and the recovery data analysis results are shown in Table 1.
[0056] Table 1
[0057]
[0058] Result analysis:
[0059] According to experimental result (seeing table 1), it can be known that the liquid chromatograph...
Embodiment 3
[0061] The only difference from Example 1 is that the mobile phase is anhydrous methanol-absolute ethanol-glacial acetic acid (500:300:25), and the pH value is adjusted to 6.4 with triethylamine.
[0062] The chromatographic conditions are: flow rate: 1.2 mL / min; column temperature: 30° C.; injection volume: 10 μL; running time: 20 minutes; detector: evaporative photodetector.
[0063] Table 2 Comparison of lysophosphatidylcholine content
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com