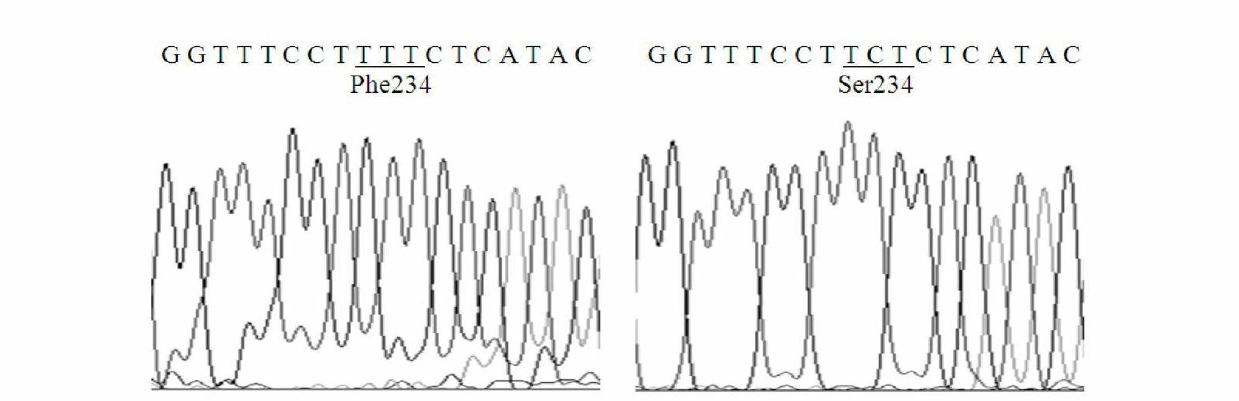
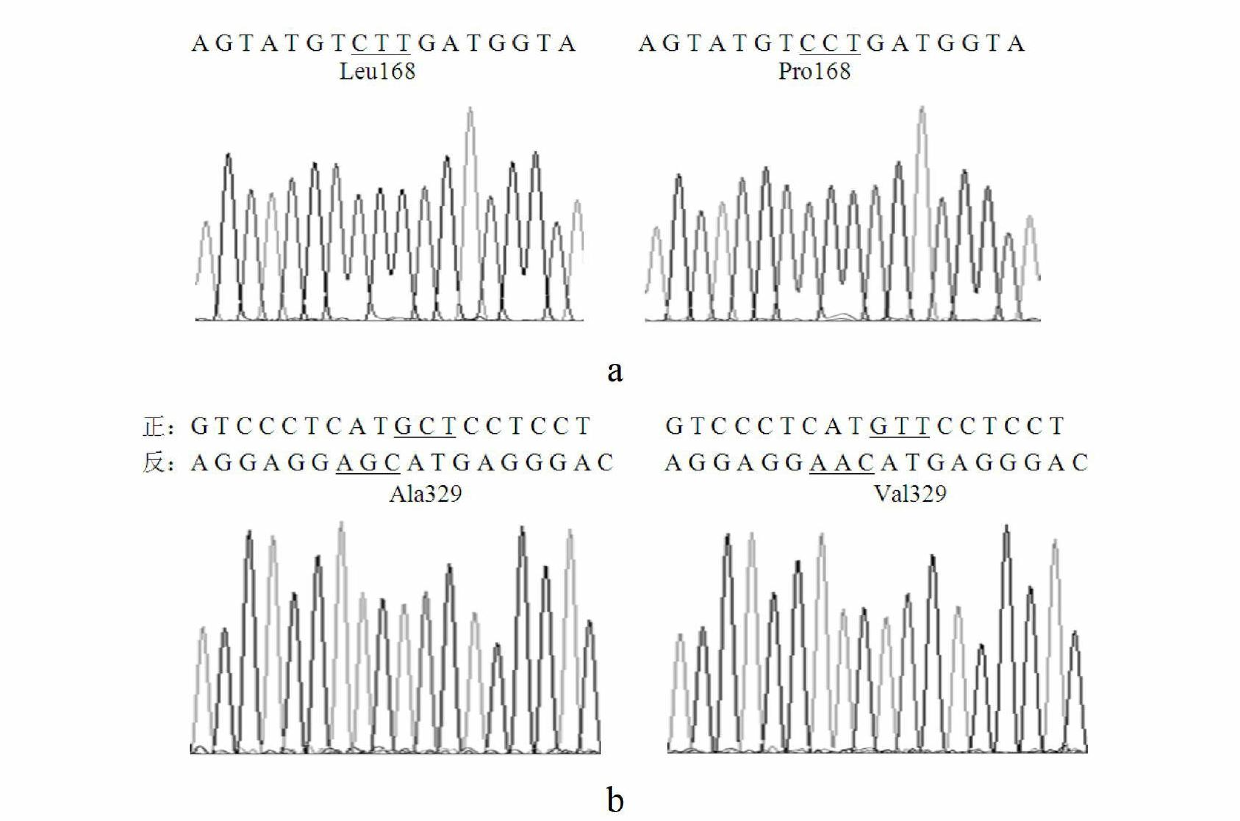
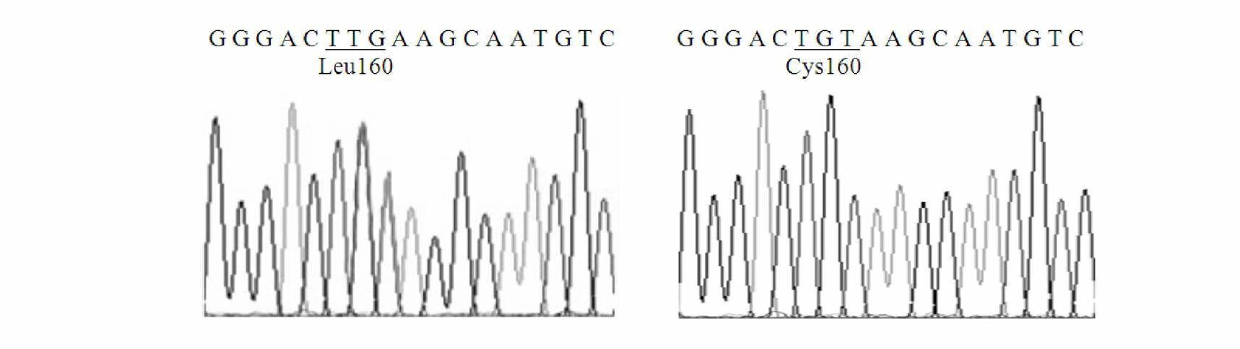
Thermal stability improved lipase mutant constructed through orthogenesis
A technology of thermal stability and directed evolution, applied in the field of genetic engineering of enzymes, can solve problems such as poor stability and achieve the effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. Construction of a Pichia pastoris library expressing lipase mutants using the error-prone PCR method
[0052] Nucleotide mutations were introduced into the lipase gene proRCL of Rhizopus sinensis in vitro by error-prone PCR. The reaction conditions for error-prone PCR are as follows:
[0053]
[0054] Wherein, the sequences of the upstream primer F and the downstream primer R are:
[0055] F: 5'-TCAAGATCCCTAGGGTTCCTGTTGGTCATAAAGGTTC-3';
[0056] R: 5'-AATTCCAGTGCGGCCGCTTACAAACAGCTTCCTTCG-3'.
[0057] PCR amplification conditions: 94°C for 3 min; 94°C for 1 min, 59°C for 1 min, 72°C for 2 min, 30 cycles; 72°C for 10 min.
[0058] After the error-prone PCR amplification product is purified by DNA purification kit, restriction endonuclease Avr II and not I respectively digest the error-prone PCR amplification product and the plasmid pPIC9K, connect to obtain the expression plasmid containing the mutant gene, and transform it into E.coli JM109 Compet...
Embodiment 2
[0060] Example 2, Construction of a Pichia pastoris library expressing lipase mutants using the DNA Shuffling method
[0061] The mutation sites in the lipase mutants constructed by error-prone PCR were randomly combined by DNA Shuffling method. The conditions for DNA shuffling are as follows:
[0062] extract easy The genome of the Pichia pastoris library expressing the lipase mutant constructed by the wrong PCR method was digested with DNase I for 30 min, and the digested product was extracted with phenol-chloroform to remove the protein and dissolved in 30 μL sterile water. Using the genome as a template, proceed as follows:
[0063] Step 1: PCR reaction system:
[0064] Taq (2.5U) 0.5 μL 5×buffer (Mg 2+ plus) 10 μL Genome 0.5 μL dNTP (25mmol / L) 4 μL dd H 2 o 34.5 μL
[0065] PCR amplification conditions: 94°C for 3 min; 10 cycles of 94°C for 1 min, 59°C for 1 min, and 72°C for 2 min; 72°C for 10 min.
[0066] Step 2: Add...
Embodiment 3
[0068] Embodiment 3, the screening of lipase mutant with high enzymatic activity
[0069] Copy the Pichia library stored on the MD plate to the MM plate, culture at 30°C for 2 days, and use the Pichia expressing the lipase of the parent Rhizopus sinensis as the control bacteria.
[0070] Plate initial screening: Add 200 μL of methanol to the cover of the MM plate every 12 hours to induce the expression of recombinant lipase. After 2-3 days of induction, heat the plate at 65°C for 60 min, cool to room temperature, and pour Fast- The blue RR dye was about 15 mL, and within 2 minutes, the black-brown colony of the single clone was darker than that of the control colony, which was the target strain of the primary screening.
[0071] 96-well plate screening method: add 300 μL of BMGY medium to a 1.8 mL / well (flat bottom) 96-well plate, and sterilize at 121 °C for 20 min. Insert the strain obtained from the primary screening into it, and use the Pichia pastoris expressing the lipas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com