Pre-fusion rsv f antigens
A pre-fusion, chimeric technology, applied in the direction of fusion peptides, viral antigen components, antiviral agents, etc., can solve the problem of not producing candidates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0535] 1. Post-fusion structure and pre-fusion model
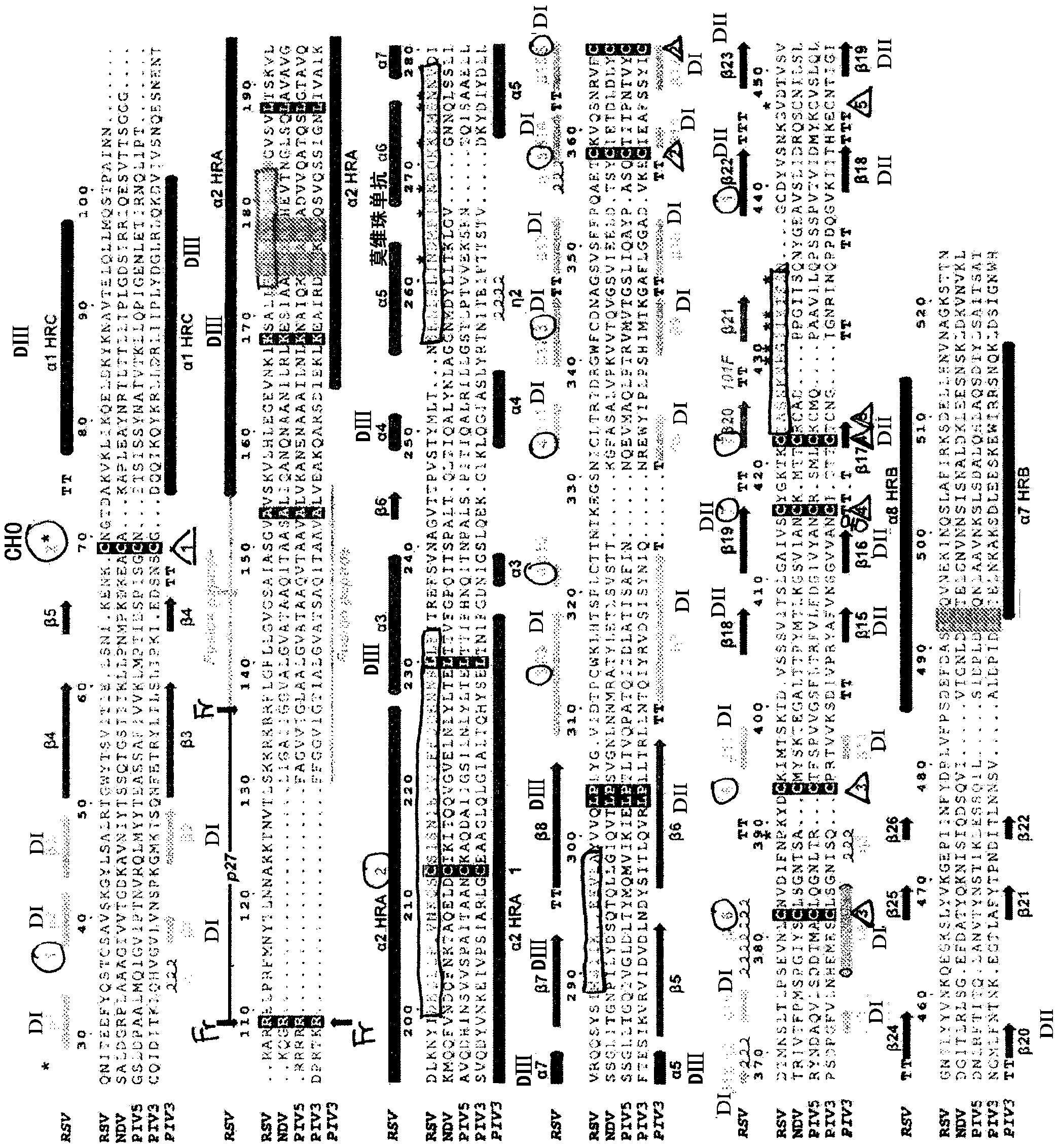
[0536] RSV F structure after fusion
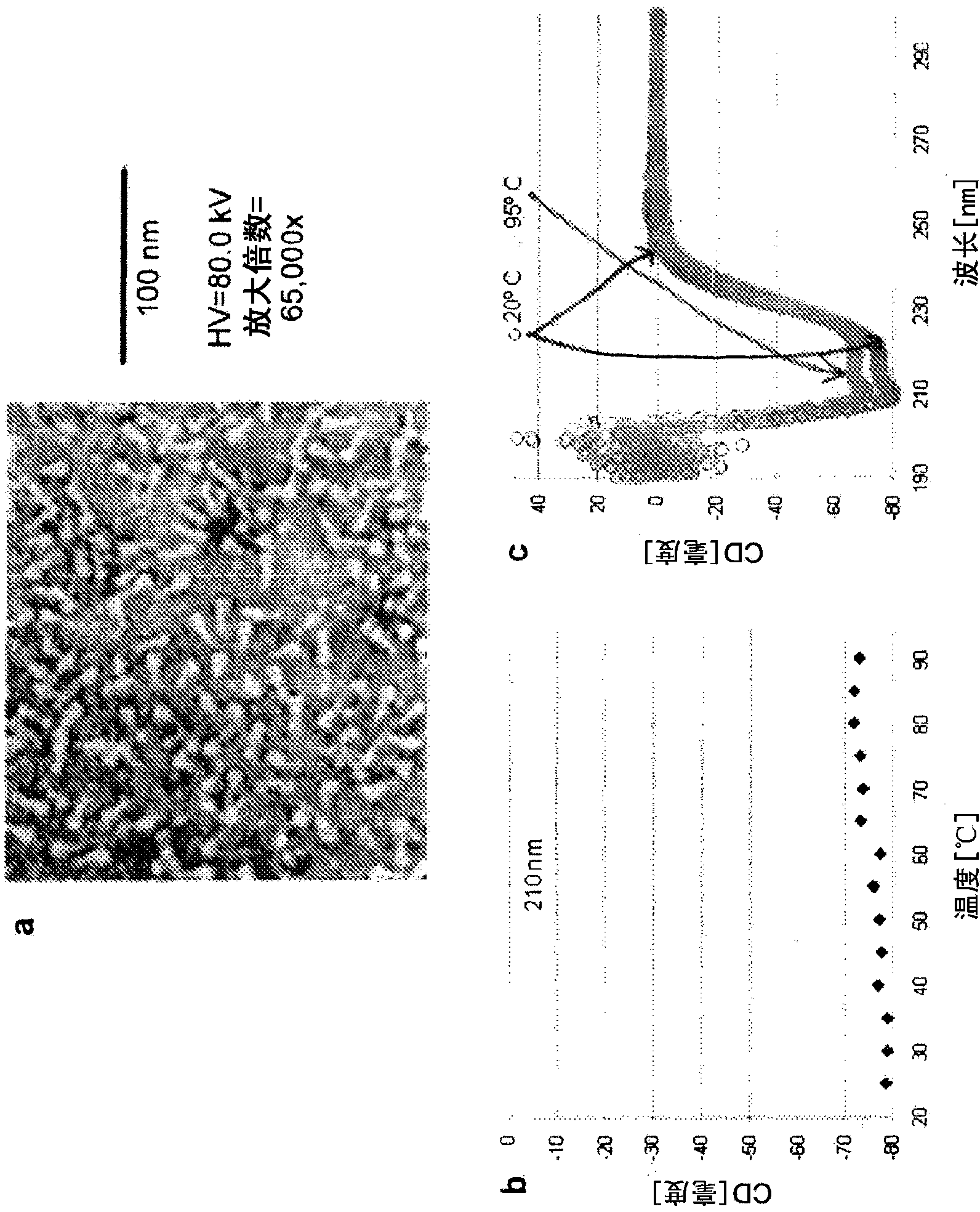
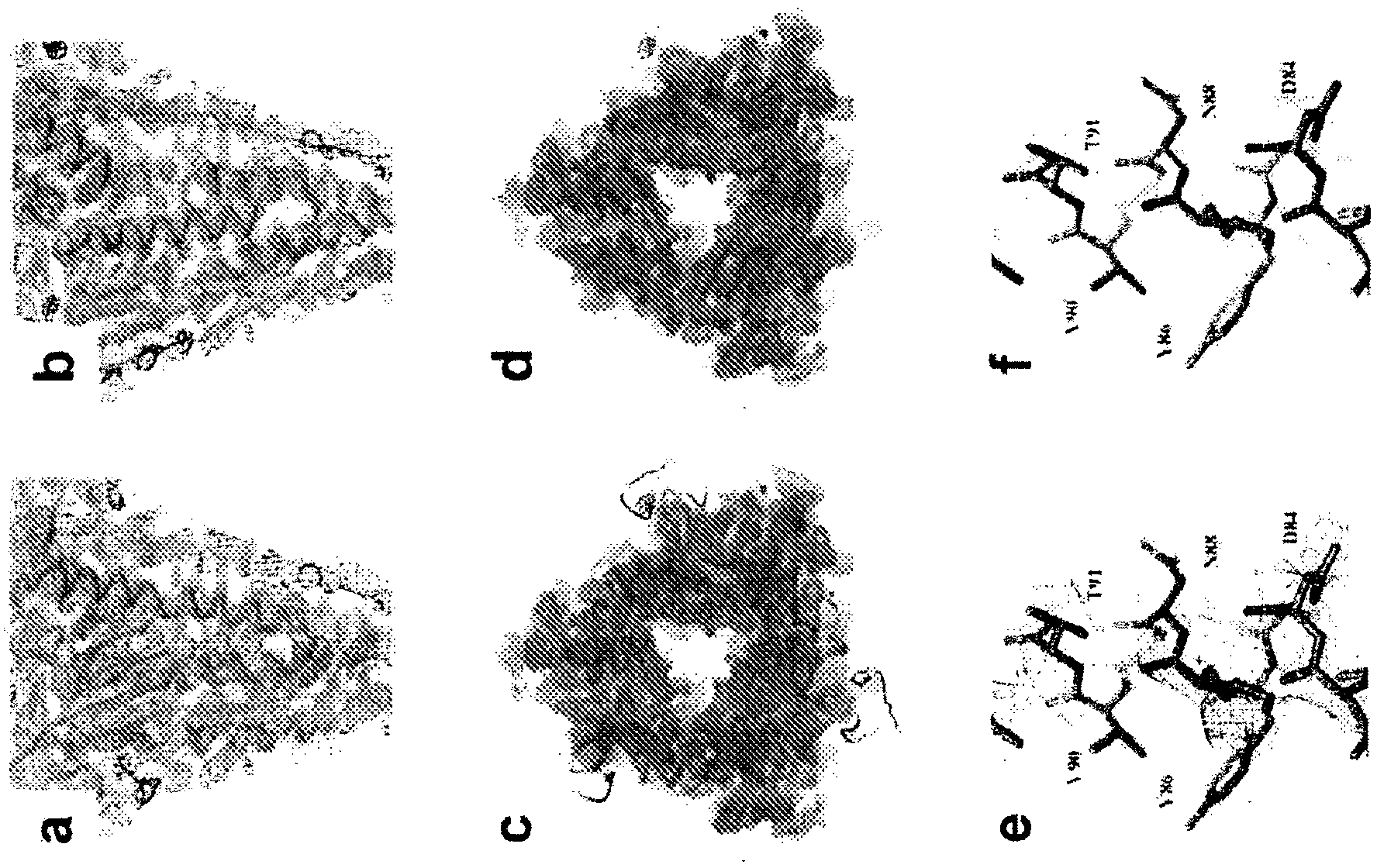
[0537] A stable, non-aggregating soluble RSV F subunit antigen ( figure 1 ). This engineered F is efficiently expressed and easily purified. Electron micrographs of negatively stained samples show the formation of non-aggregated, uniform cane-shaped molecules, consistent with postfused F trimers ( figure 2 A). The engineered F trimer is stable, and the circular dichroism spectrum shows no obvious melting when the temperature is up to 95 °C ( figure 2 B and 2C).
[0538] The RSV F protein was crystallized, and its structure was determined by molecular substitution and three-fold non-crystallographic symmetry (NCS) averaging (Table 3 and image 3 ). The structure does not include the p27 fragment (peptide between the two furin sites, which is lost after cleavage), the fusion peptide, the transmembrane region and the cytoplasmic domain ( Figure 4 ).
[0539] Table 3. Crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com