Hexacyanoferrate battery electrode modified with ferrocyanides or ferricyanides
A battery electrode and hexacyanohydrin technology, applied in the field of electrochemical batteries, can solve the problems of poor dissolution of TMHCF and shortened cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
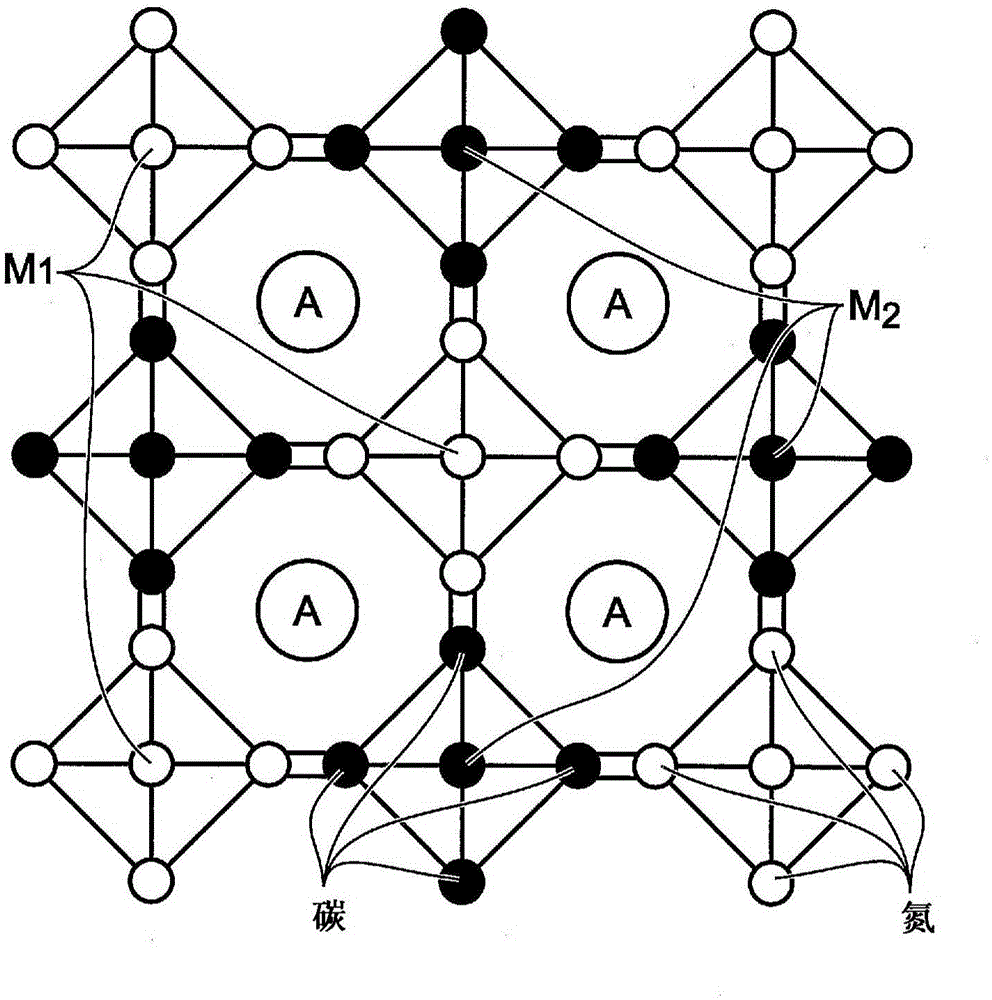
[0043] Disclosed herein is the use of ferrocyanide or ferricyanide as an additive in rechargeable batteries having transition metal hexacyanoferrate (TMHCF) electrodes, which improves the performance of said electrodes in non-aqueous electrolytes. Ferrocyanide or ferricyanide A x Fe(CN) 6 (x=3 or 4) dissociates into A + and Fe(CN) 6 3- or Fe(CN) 6 4- ion. These ions are able to push Equation 2 back, which prevents TMHCF from dissolving in non-aqueous electrolytes:
[0044] A x m y Fe z (EN) n .mH 2 O=xA a+ +y M b- +[Fe z (CN) n ] c- +mH 2 O. (2)
[0045] TMHCF can be expressed as A x m y Fe z (EN) n .mH 2O, A is selected from alkali metal or alkali metal, and wherein M can be one or several transition metals. As an additive, ferrocyanide or ferricyanide increases the capacity of TMHCF and its capacity retention.
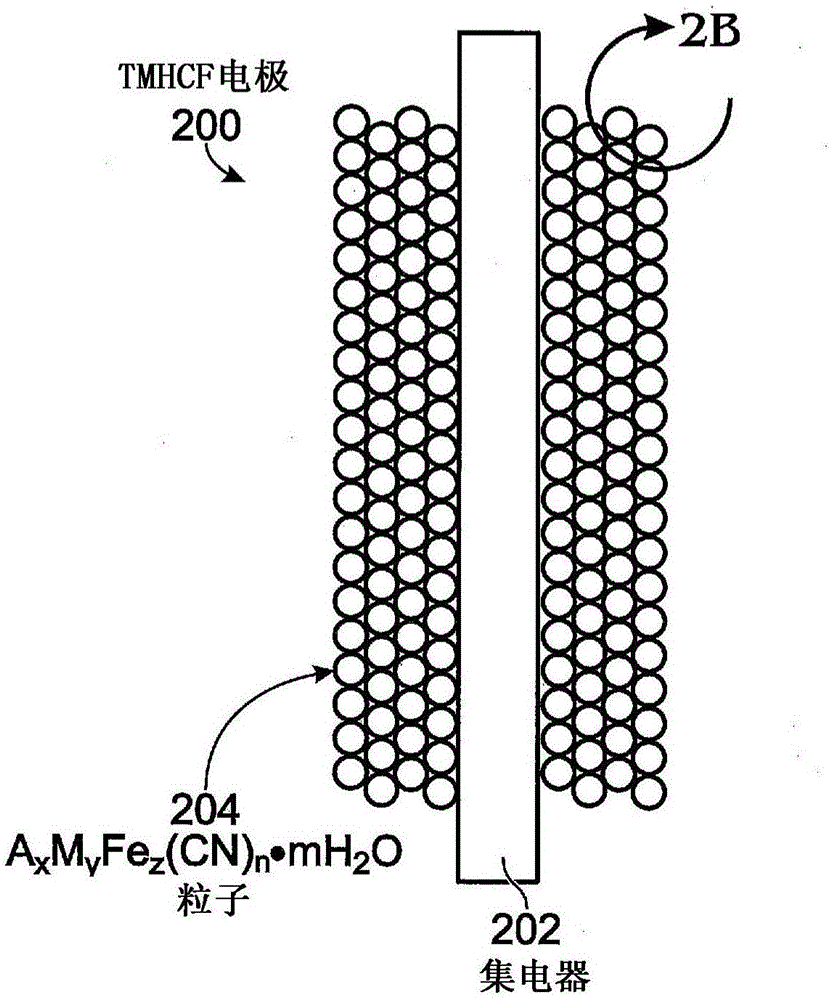
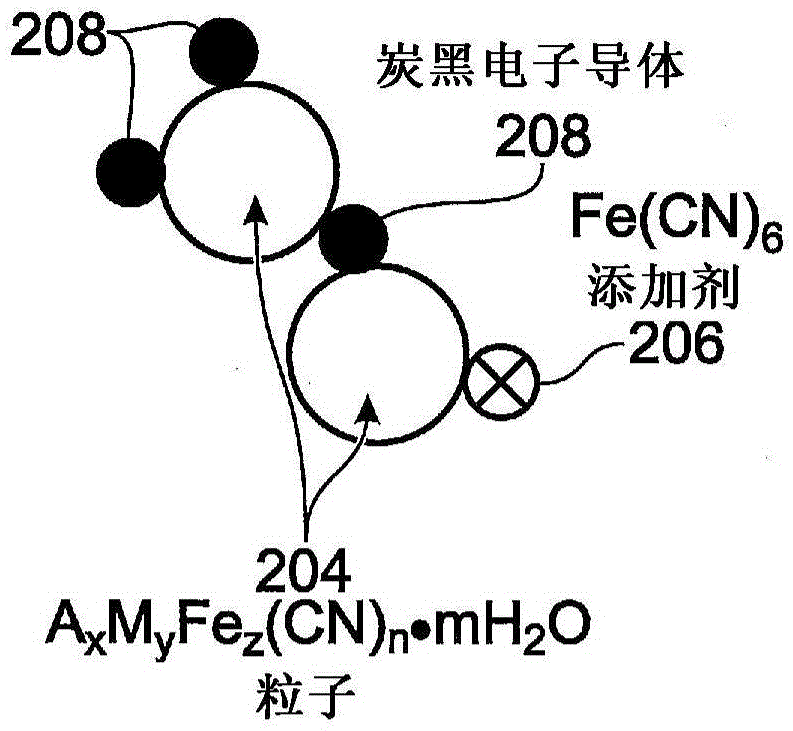
[0046] Therefore, TMHCF battery electrodes have Fe(CN) 6 additive. The electrodes are covered by a current collector A x m y Fe z (EN)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com