Rechargeable metal-air redox flow battery combining electrochemical preparation
A metal-air and liquid flow battery technology, which is applied in the fields of power engineering and electrochemical energy storage, can solve the problems of cross-contamination between cathode and anode, serious side reactions, and waste of electric energy, and achieve low energy consumption, high energy density and power density , good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
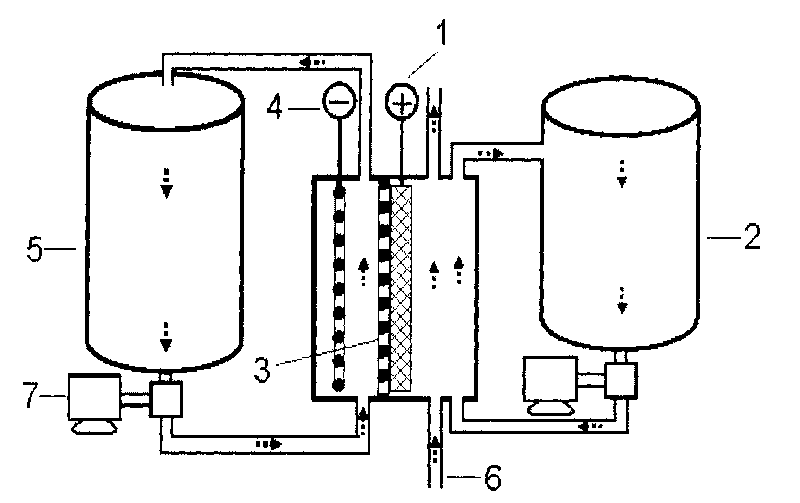
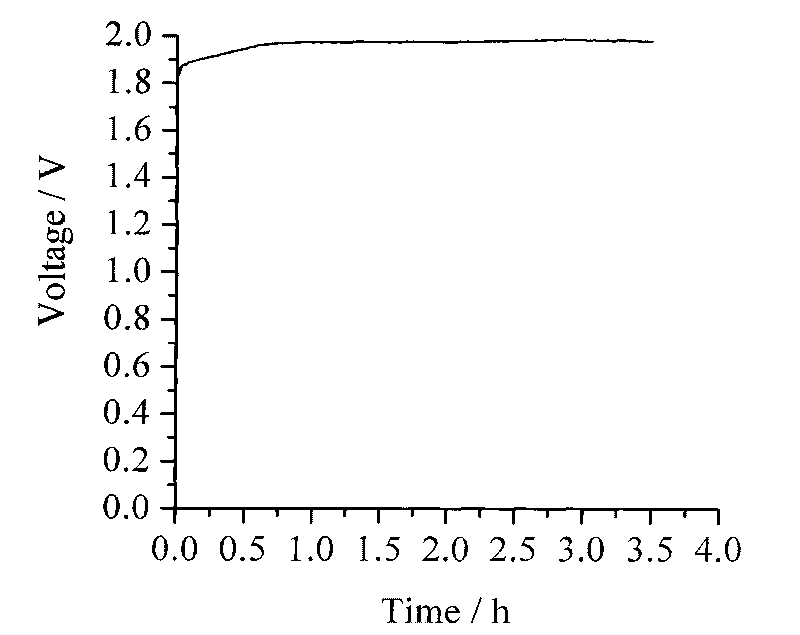
[0023] The base material of the negative electrode is Ni sheet (as current collector and negative base), the positive electrode is sintered nickel oxide electrode, and the positive / negative electrode is separated by Nafion115 cation exchange membrane to assemble the battery. The positive electrode electrolyte is an aqueous solution of 35mL of 0.1M n-propanol+7MKOH, wherein n-propanol is a reducing raw material, water is a solvent, potassium hydroxide is a supporting electrolyte, and the negative electrode electrolyte is an aqueous solution of 35mL of 0.4MZnO+7MKOH, wherein Zinc ions are depositable metal ions, water is the solvent, potassium hydroxide is the supporting electrolyte, the anode electrolyte and the anode electrolyte are stored in external glass containers respectively, and each is fed into the positive and negative electrode areas of the battery by a magnetic circulation pump . When charging, the zinc reduction deposition reaction occurs at the negative electrode ...
Embodiment 2
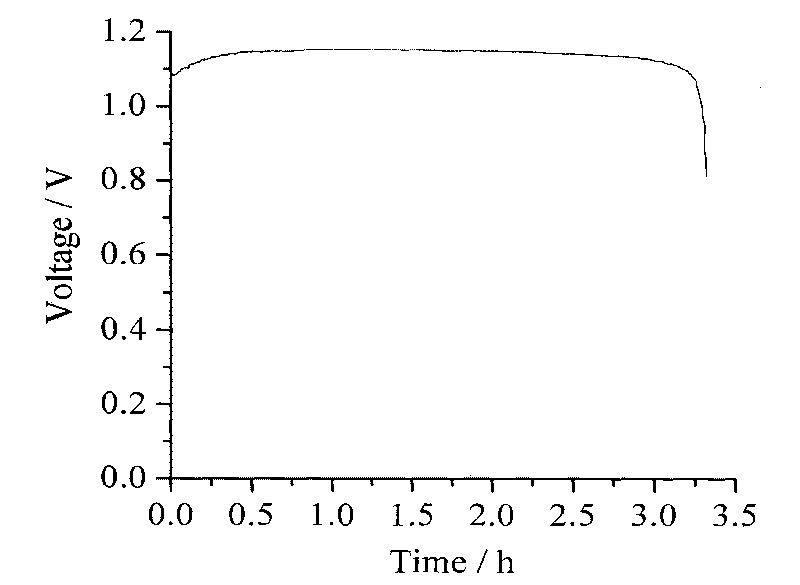
[0025] Copper foil is used as the negative electrode (as current collector and negative electrode matrix), ZnO-saturated 6M KOH aqueous solution is the negative electrode electrolyte, in which zinc ions are depositable metal ions, water is the solvent, potassium hydroxide is the supporting electrolyte, Asahi Kasei F4602 type cation exchange The membrane is a diaphragm, with a bifunctional oxygen electrode as the positive electrode (which can not only catalyze the reduction of oxygen, but also produce oxygen electrochemically), and use 1.7M Na 2 CO 3 The aqueous solution is the positive electrode electrolyte, where Na 2 CO 3 It is a supporting electrolyte, and water is both a solvent and a reducing raw material (the positive reaction is to electrolyze water to generate protons). Negative electrode area, at 20mA / cm 2 The current density is charged and electrolyzed, electrolyzed for 12 hours, and NaHCO is obtained in the positive electrode area 3 solution, deposit metal zinc ...
Embodiment 3
[0027] Both positive and negative electrodes use graphite plates as current collectors and porous graphite felt as electrode materials. The positive and negative electrodes are separated by Nafion115 cation exchange membranes and assembled into batteries. The positive electrode electrolyte is 35mL of 0.1M cystine + 3M HBr aqueous solution, in which cystine is the reducing raw material, water is the solvent, HBr is the supporting electrolyte and oxidation medium (this electrode reaction is indirect electrochemical oxidation, that is, HBr electrooxidation into Br 2 , Br 2 Oxidation of cystine to L-sulfoalanine, Br 2 Regenerate HBr), negative electrode electrolyte is 35mL of 0.5M CdSO 4 +3MH 2 SO 4 aqueous solution, where Cd 2+ are metal ions that can be deposited electrochemically, water is the solvent, and H 2 SO 4 In order to support the electrolyte, the positive electrode electrolyte and the negative electrode electrolyte are stored in the external glass container resp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com