Tin-antimony oxide coating titanium anode material for electrosynthesis of ferrate
A technology of oxide coating and ferrate, which is applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the hidden dangers of batteries with sealed structure, low generation potential, carcinogenicity and other problems, and achieve simple and mature preparation process and technology, mechanical strength High, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
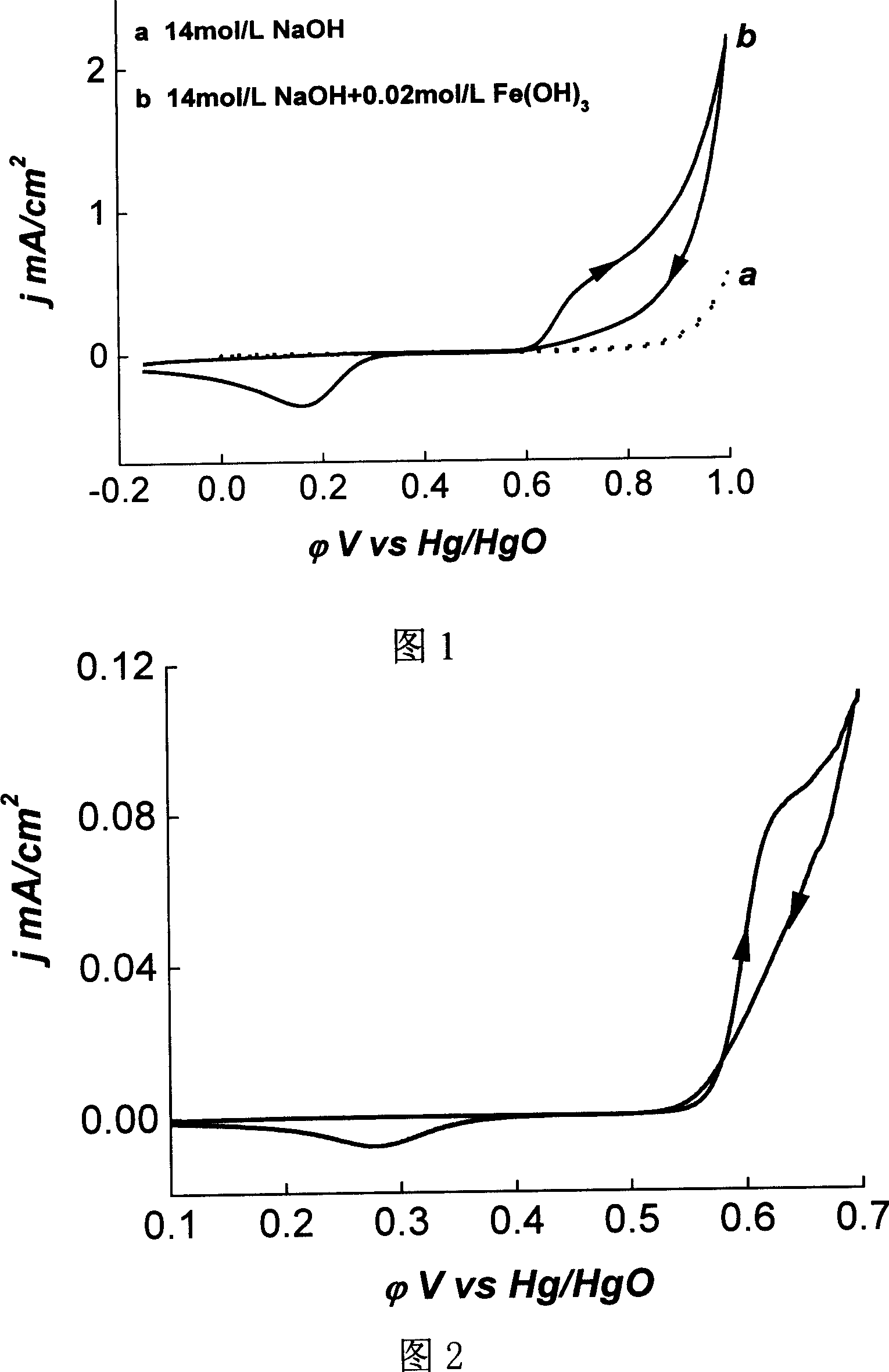
[0026] Tin antimony oxide coating anode material: the synthesis condition is Sn:Sb=57~5:1 (mol ratio), after drying by infrared lamp and burning at 150~1050°C. respectively containing Fe(OH) 3 and does not contain Fe(OH) 3Cyclic voltammogram in 14mol / L NaOH solution. The scanning speed adopted is 50mV / s, which is equivalent to the working mode of fast charging and discharging (the time to complete a charging and discharging cycle is 46 seconds. (Note: the scanning in the direction of potential increase is relative to charging, that is, ferrate is generated; the opposite Direction scanning relative to discharge, ferrate reduction). The Fe electrode is the counter electrode, and the reference electrode is the Hg / HgO electrode of the same solution. Under this working mode, the ferrate is almost regenerated under its thermodynamic equilibrium condition, that is, the charging process is very Rapid, suitable for the working mode of fast charging. Or it is considered that the disch...
Embodiment 2
[0028] The synthesis conditions of the above-mentioned coated anode material are slightly different from those in Example 1, and the synthesis conditions are Sn:Sb=56˜3:1 (mol ratio). Slow scanning is still carried out in the above-mentioned similar strong alkaline solution, which is equivalent to the working mode of slow charging and discharging. It takes 1.8 hours to complete a charge-discharge cycle. During the charge-discharge process, the electrochemical oxidation / reduction cycle of ferrate is highly reversible, with a difference of 110mV, which is favorable from the perspective of energy conversion.
Embodiment 3
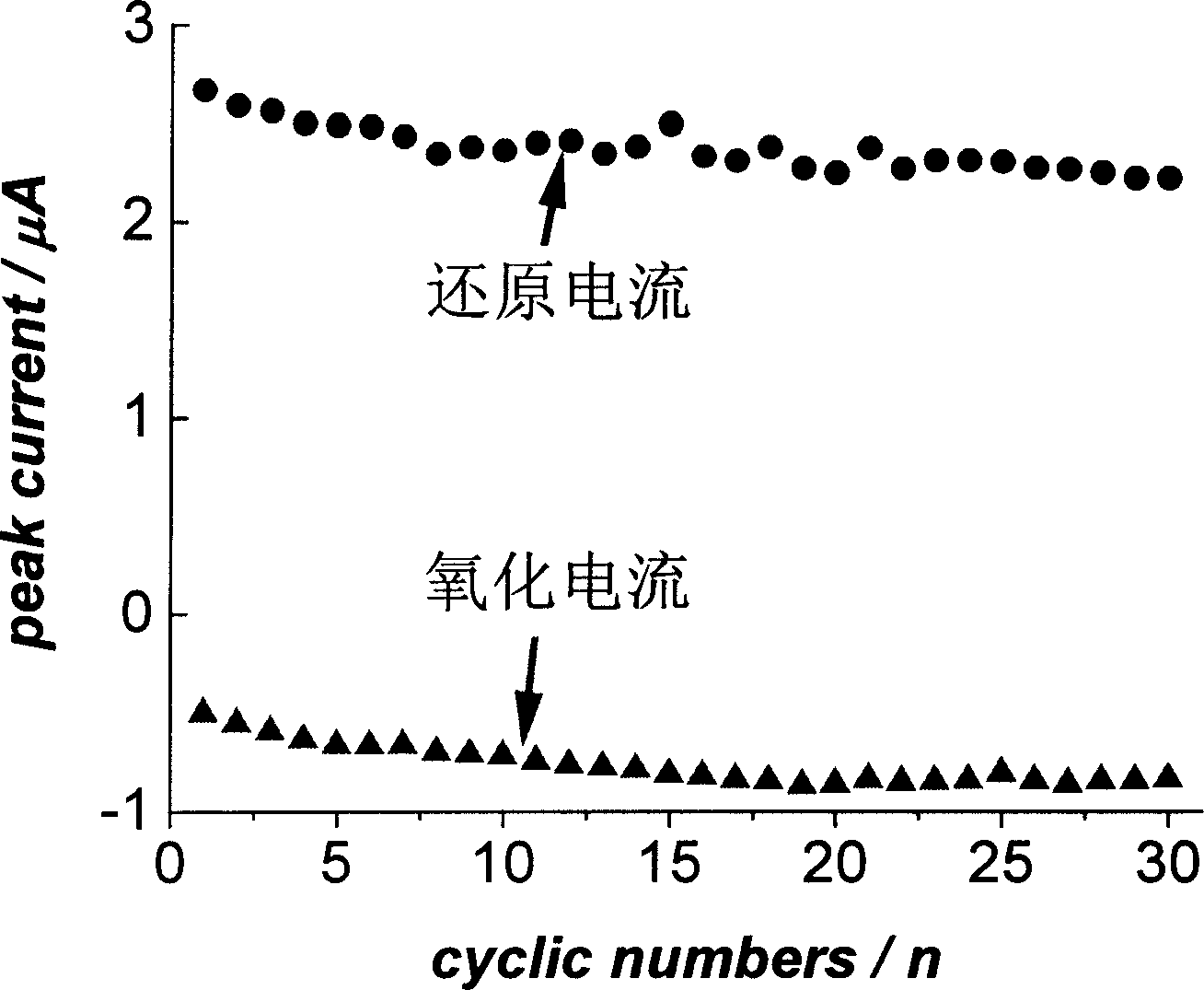
[0030] Carry out 30 charge-discharge cycles (or redox cycles) with the coated anode materials used in the above two implementation examples in a similar strong alkaline solution, and then measure the oxidation current and reduction current to measure the coating anode material The results show that the material is very stable, that is, resistant to electrochemical corrosion and strong oxidant corrosion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com