Catalyst for electrochemically synthesizing ammonia and preparing method thereof
A technology for synthesizing ammonia and catalysts, applied in physical/chemical process catalysts, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc. The effect of reducing consumption and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
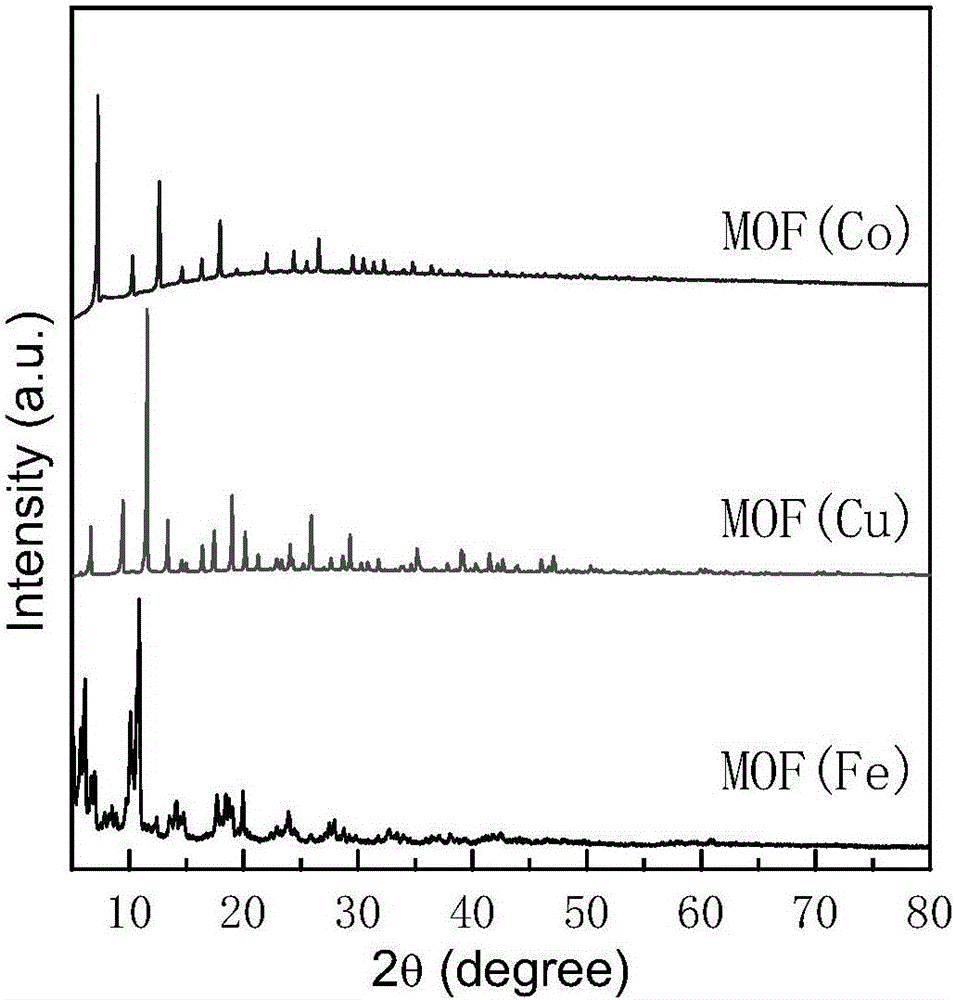
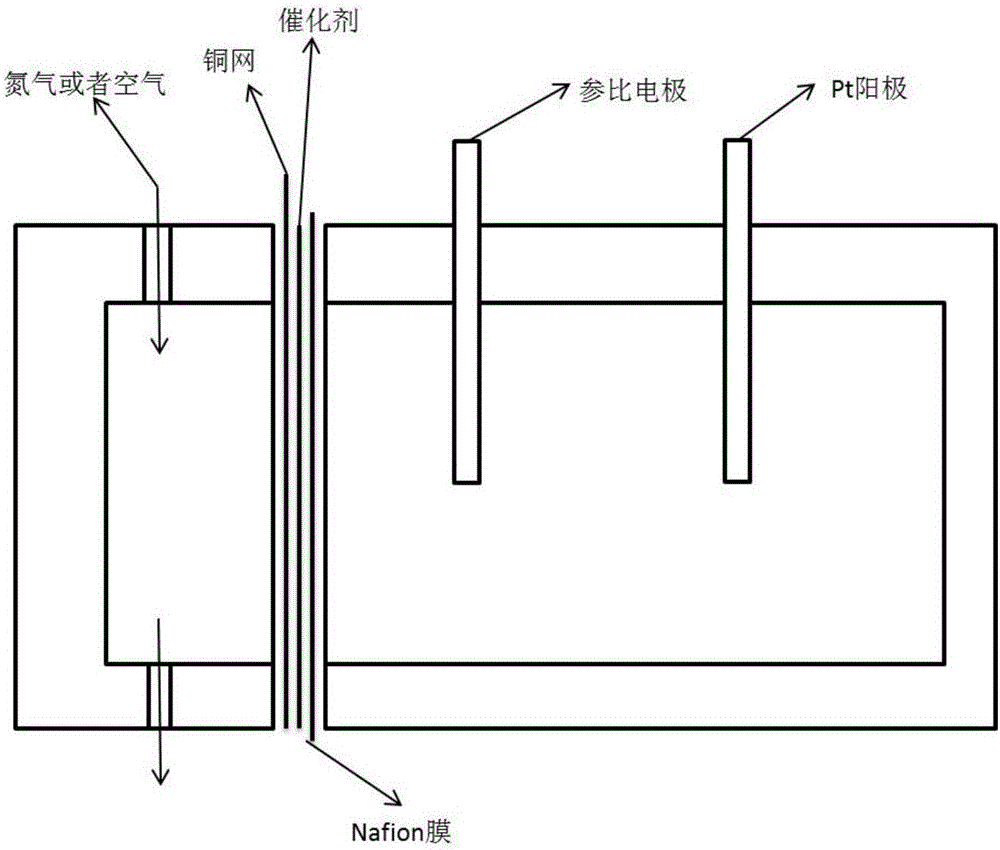
[0024] MOF(Cu) is selected as the metal-organic framework material, and the synthesis step is Cu(NO 3 ) 2 ·3H 2 O, 1,3,5-BTC, according to a certain molar ratio (Cu 2+ :1,3,5-BTC:H 2 O=3:6:2) were dissolved in 180ml of water and 180ml of absolute ethanol respectively, and after stirring for 1 hour until uniform, the Cu(NO 3 ) 2 ·3H 2 O solution was dropped into 1,3,5-BTC solution and stirred for 2 hours. The mixed solution was then transferred to a polytetrafluoroethylene-lined jelly, then loaded into a stainless steel hydrothermal jelly, and placed in an oven, kept at 120°C for 24 hours and then cooled to room temperature. The product was filtered and washed, and the final product was dried in a vacuum oven at 60°C to obtain a MOF (Cu) sample, whose XRD pattern is as follows figure 1 shown. With the mass ratio of MOF(Cu):graphite:nafion=80:15:5, where the concentration of nafion is 80wt%, mix it with absolute ethanol to make a slurry, and evenly coat it on 4cm 2 On h...
Embodiment 2
[0026] MOF(Cu) is selected as the metal-organic framework material, and the synthesis step is Cu(NO 3 ) 2 ·3H 2 O, 1,3,5-BTC, according to a certain molar ratio (Cu 2+ :1,3,5-BTC:H 2 O=3:6:2) were dissolved in 180ml of water and 180ml of absolute ethanol respectively, and after stirring for 1 hour until uniform, the Cu(NO 3 ) 2 ·3H 2 O solution was dropped into 1,3,5-BTC solution and stirred for 2 hours. The mixed solution was then transferred to a polytetrafluoroethylene-lined jelly, then loaded into a stainless steel hydrothermal jelly, and placed in an oven, kept at 120°C for 24 hours and then cooled to room temperature. The product was filtered and washed, and the final product was dried in a vacuum oven at 60°C to obtain a MOF (Cu) sample, whose XRD pattern is as follows figure 1 shown. With the mass ratio of MOF (Cu): acetylene black: polytetrafluoroethylene: = 85:13.5:1.5, wherein the concentration of polytetrafluoroethylene is 5wt%, mixed evenly with absolute e...
Embodiment 3
[0028] The metal-organic framework material is MOF(Fe), and the synthesis step is Fe(NO 3 ) 3 9H 2 O, 1,3,5-BTC, 5mol / LHF and water according to a certain molar ratio (Fe 3+ :1,3,5-BTC:HF:H 2 (0=1.0:0.66:2.0:280) were mixed and stirred for 3-4 hours. The mixture was then transferred to a polytetrafluoroethylene-lined box, loaded into a stainless steel hydrothermal box, placed in an oven, and reacted at 150 °C for 84 hours before cooling to room temperature. The product was filtered and washed, and the final product was dried in a vacuum oven at 60°C to obtain a MOF(Fe) sample, whose XRD pattern is as follows figure 1 shown. With the mass ratio of MOF(Fe):carbon black:nafion:=80:1:5, wherein the concentration of nafion is 90wt%, mixed with absolute ethanol to make a slurry, uniformly coated on 4cm 2 On hydrophobic carbon paper, dry at 100°C for 8h, used for electrochemical synthesis of ammonia experiment, the reaction temperature is 80°C, the voltage is -1.2V, the synthes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com