Silver-copper nano alloy and electrosynthesis method thereof
A nano-alloy, silver-copper technology, which is applied in the field of silver-copper nano-alloy and its capillary force-induced electrochemical synthesis, which can solve the problems of application limitations, inability to meet large-scale industrial applications, and complicated preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
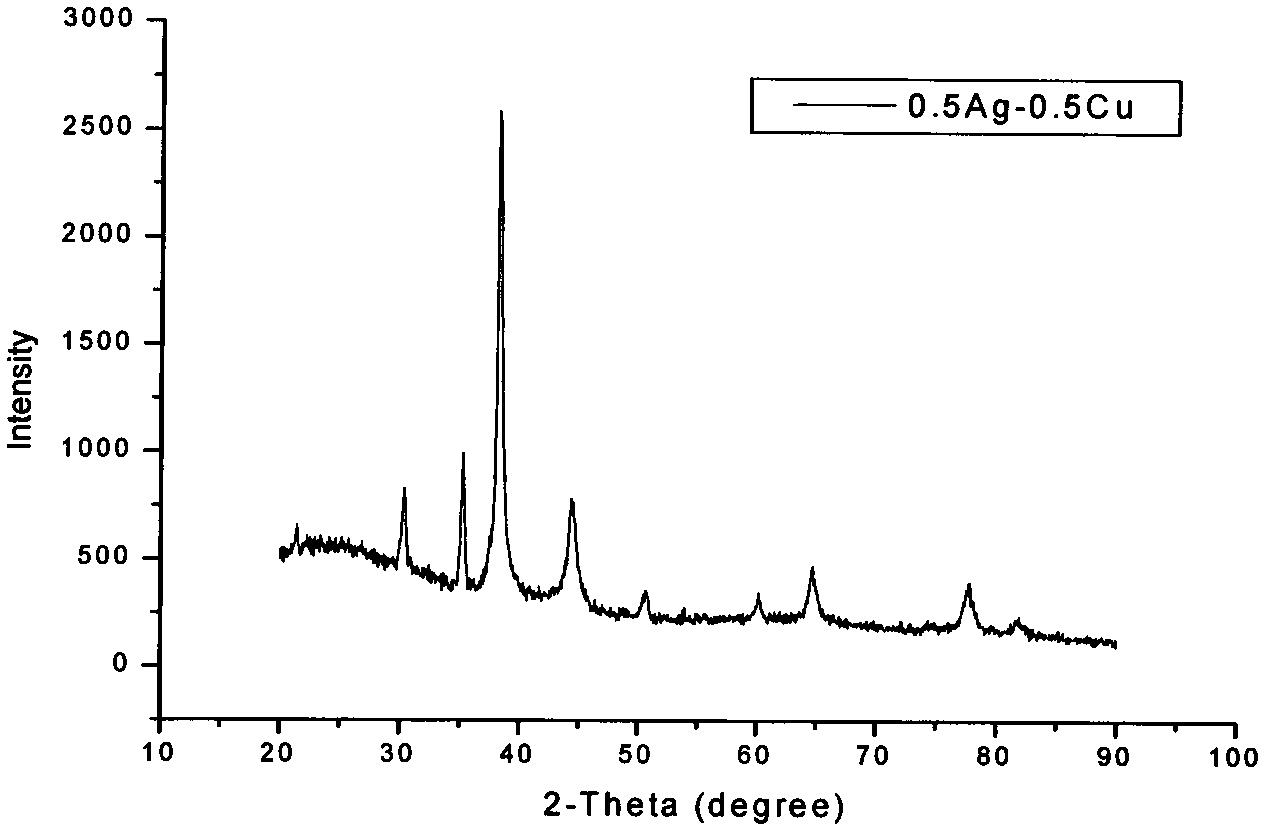
[0031] The silver-copper nano-alloy prepared in this embodiment includes Ag and Cu, and its molar composition is 0.5Ag-0.5Cu. The phase of silver-copper nano-alloy is FCC structure silver single-phase solid solution, the (111) diffraction peak of the alloy is consistent with the (111) diffraction peak of pure metal Ag, and the alloy has a surface plasmon resonance peak. The shape of the silver-copper nano-alloy is a dendrite, the distance between the secondary dendrites is 100nm, and the length of the tertiary dendrites is 100-200nm.
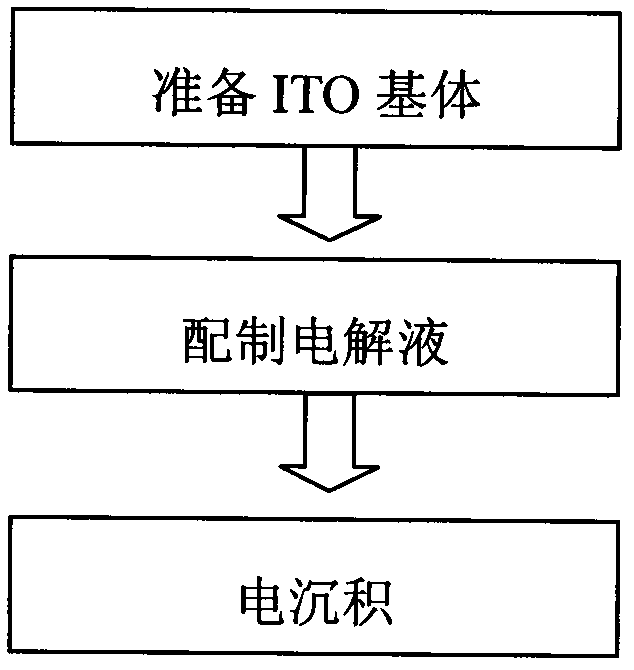
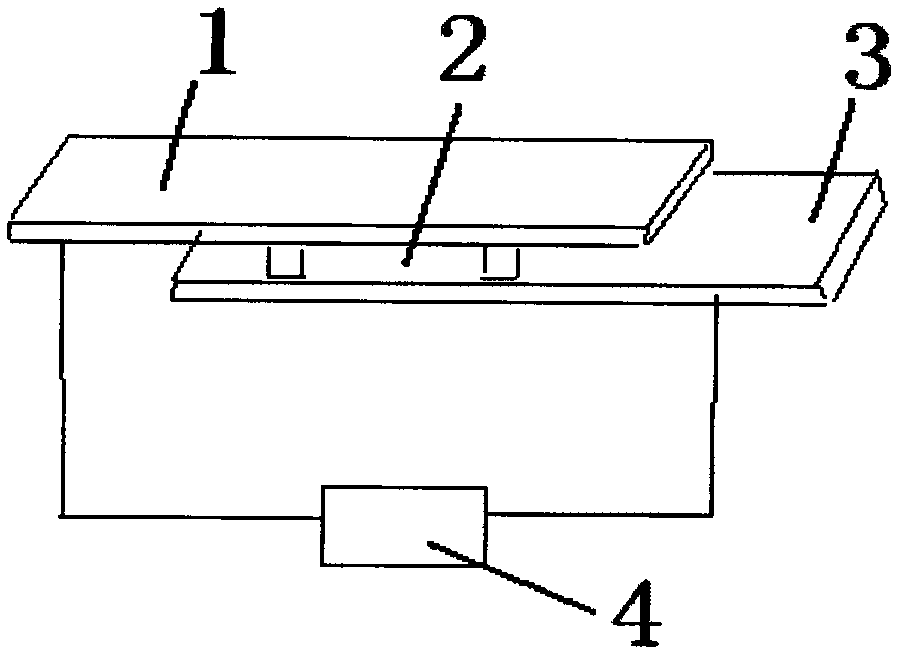
[0032] The electrode used in the preparation of the silver-copper nano-alloy in this embodiment uses the ITO glass plate 1 as the cathode and the silver plate 3 as the anode, and the dimensions of the ITO glass plate in all directions are consistent with the size of the silver electrode. When assembling, place the ITO glass plate 1 and the silver plate 3 horizontally, and make the conductive surface of the ITO glass plate 1 face down, and make t...
Embodiment 2
[0042] The silver-copper nano-alloy prepared in this embodiment includes Ag and Cu, and its molar composition is 0.8Ag-0.2Cu. The phase of silver-copper nano-alloy is silver single-phase solid solution with FCC structure, the (111) diffraction peak of the alloy is consistent with that of pure metal Ag, and the alloy has a surface plasmon resonance peak. The shape of the silver-copper nano-alloy is a dendrite, the distance between the secondary dendrites is 200nm, and the length of the third dendrites is 100-300nm.
[0043] The electrodes used in the preparation of silver-copper nano-alloys in this embodiment use the ITO glass plate 1 as the cathode and the silver plate 3 as the anode, and the dimensions in all directions of the ITO glass plate are consistent with the size of the silver electrode. When assembling, place the ITO glass plate 1 and the silver plate 3 horizontally, and make the conductive surface of the ITO glass plate 1 face down, and make the ITO conductive surfa...
Embodiment 3
[0053] The silver-copper nano-alloy prepared in this embodiment includes Ag and Cu, and its molar composition is 0.9Ag-0.1Cu. The phase of silver-copper nano-alloy is silver single-phase solid solution with FCC structure, the (111) diffraction peak of the alloy is consistent with that of pure metal Ag, and the alloy has a surface plasmon resonance peak. The shape of the silver-copper nano-alloy is a dendrite, the distance between the secondary dendrites is 200-300nm, and the length of the third dendrites is 300-500m.
[0054] The electrodes used in the preparation of silver-copper nano-alloys in this embodiment use the ITO glass plate 1 as the cathode and the silver plate 3 as the anode, and the dimensions in all directions of the ITO glass plate are consistent with the size of the silver electrode. When assembling, place the ITO glass plate 1 and the silver plate 3 horizontally, and make the conductive surface of the ITO glass plate 1 face down, and make the ITO conductive su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com