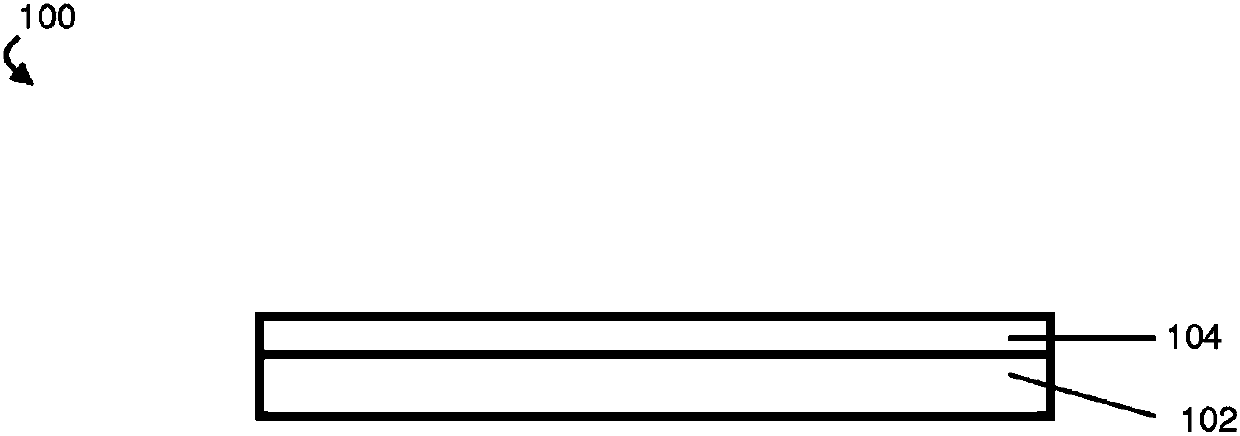
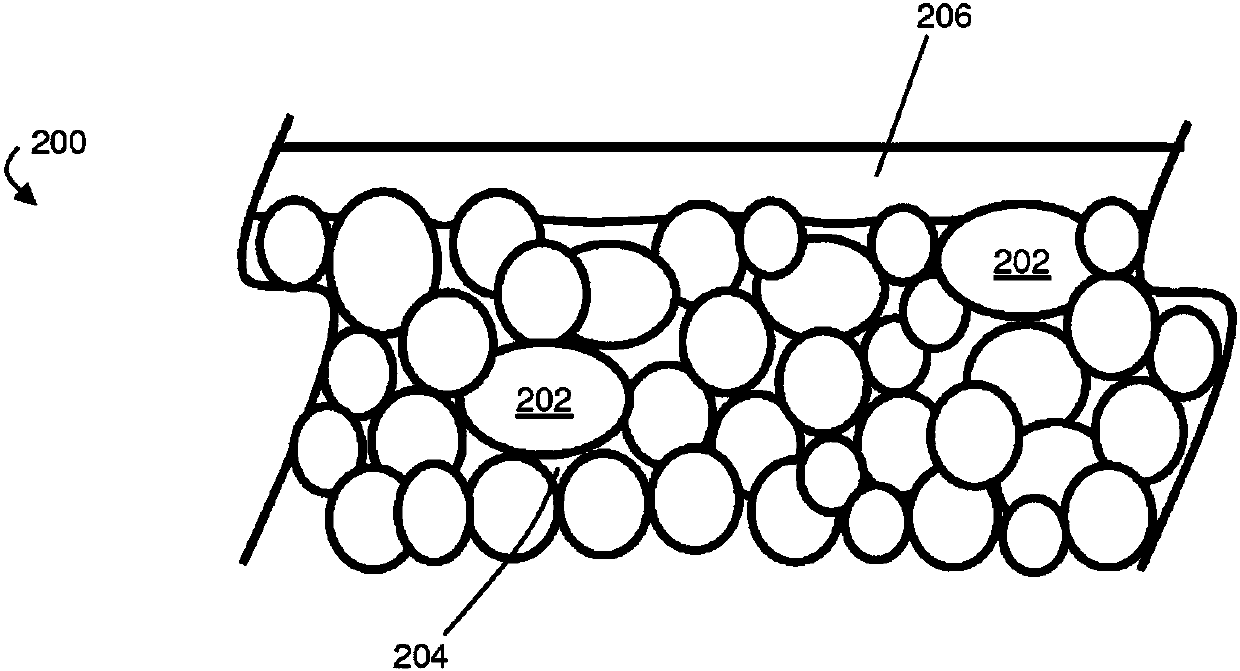
Protective layers in lithium-ion electrochemical cells and associated electrodes and methods
An electrochemical, lithium-ion technology, applied in the field of protective layers and related electrodes and methods in lithium-ion electrochemical cells, can solve problems such as unfavorable interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] This example describes the fabrication and testing of an electrochemical cell comprising an anode, a cathode coated with a lithium ion conducting ceramic, and a porous separator disposed between the anode and cathode.
[0140] In the electrochemical cell, the anode was graphite and 10 μm Cu foil served as substrate and current collector. The porous separator was a 25 μm thick polyolefin layer (Celgard 2325). The cathode is lithium iron phosphate (LFP) coated on an aluminum substrate. The capacity of the cathode is about 1.21mAh / cm 2 . Lithium oxide was coated onto the LFP cathode by vacuum deposition, where an oxygen-containing gas reacted with lithium vapor. In both anode and cathode PVDF was used as binder.
[0141] The above assemblies were assembled in a single layer cell with a separator placed between the anode and cathode, and the cell assembly was placed in a foil pouch. The total active cathode surface area is 16.574cm 2 . 0.3 mL of LP30 electrolyte (44....
Embodiment 2
[0149] This example describes the fabrication and testing of an electrochemical cell comprising a sulfur oxide coated LFP cathode.
[0150] The materials and procedures given in Example 1 were used and followed except that the LFP cathode was coated with sulfur oxide instead of lithium oxide. A 0.5 μm thick oxysulfide ceramic coating was sputtered on the LFP electrodes. Figure 10 An improved discharge capacity fade rate is shown in the battery (1000) comprising the sulfur oxide coated LFP relative to the control battery of Comparative Example 1 (1020).
Embodiment 3
[0152] This example describes the fabrication and testing of an electrochemical cell comprising a lithium oxide-coated LFP cathode.
[0153] A 2 μm thick lithium oxide layer was vacuum deposited on the LFP electrode. A battery was constructed in the same manner as in Example 1 and cycled at room temperature for 5 cycles. Fully charged cells were stored at 60 °C for one week and then cycled at room temperature. Control cells with conventional LFP cathodes were constructed and cycled / stored in the same manner. Such as Figure 11 As shown, 100% self-discharge was observed from the control cell (1120) of Comparative Example 1. In the presence of a lithium oxide ceramic coating on the LFP cathode, the cell self-discharge was reduced to 56% (1100).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Surface roughness | aaaaa | aaaaa |
| Surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com