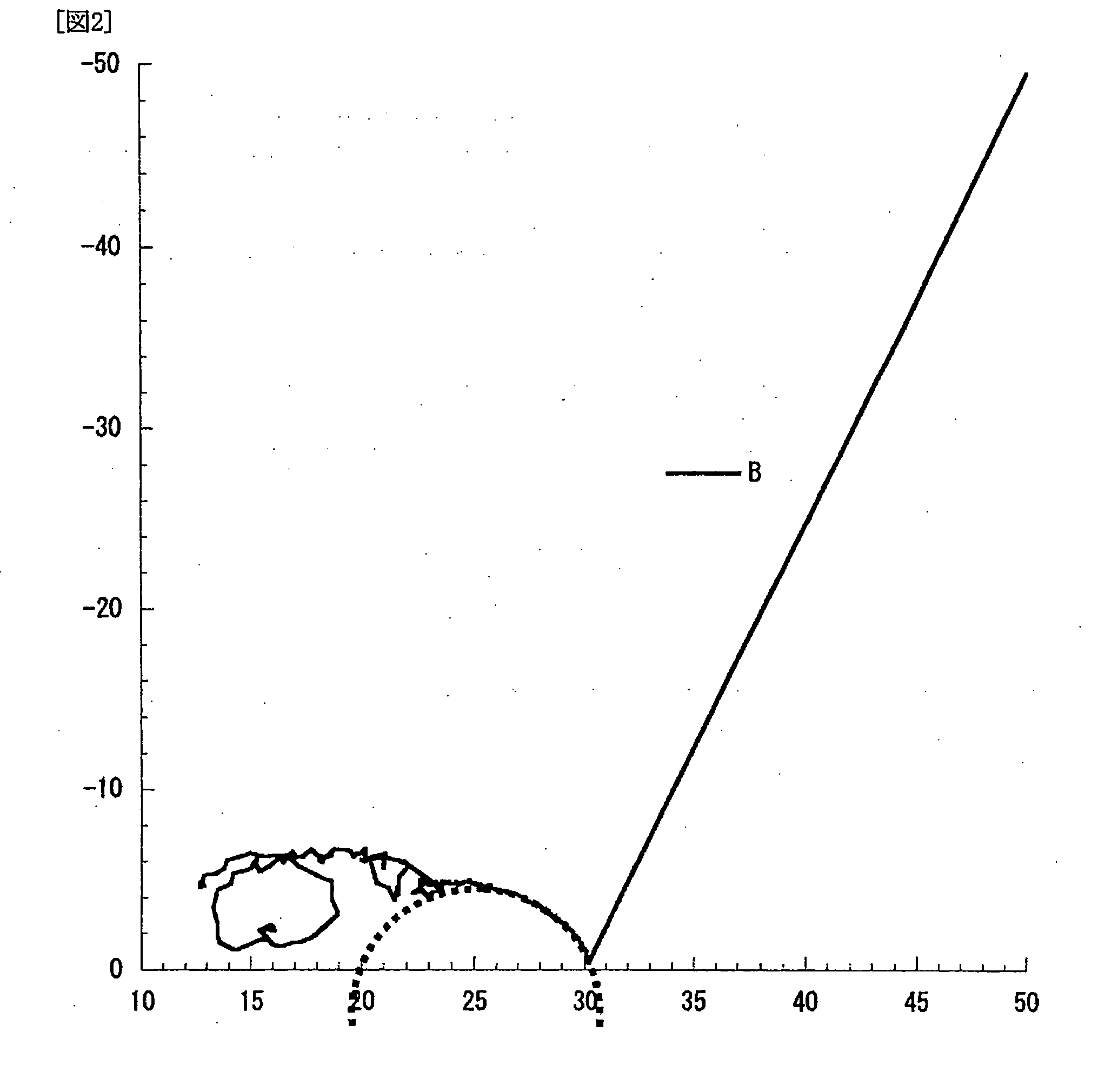
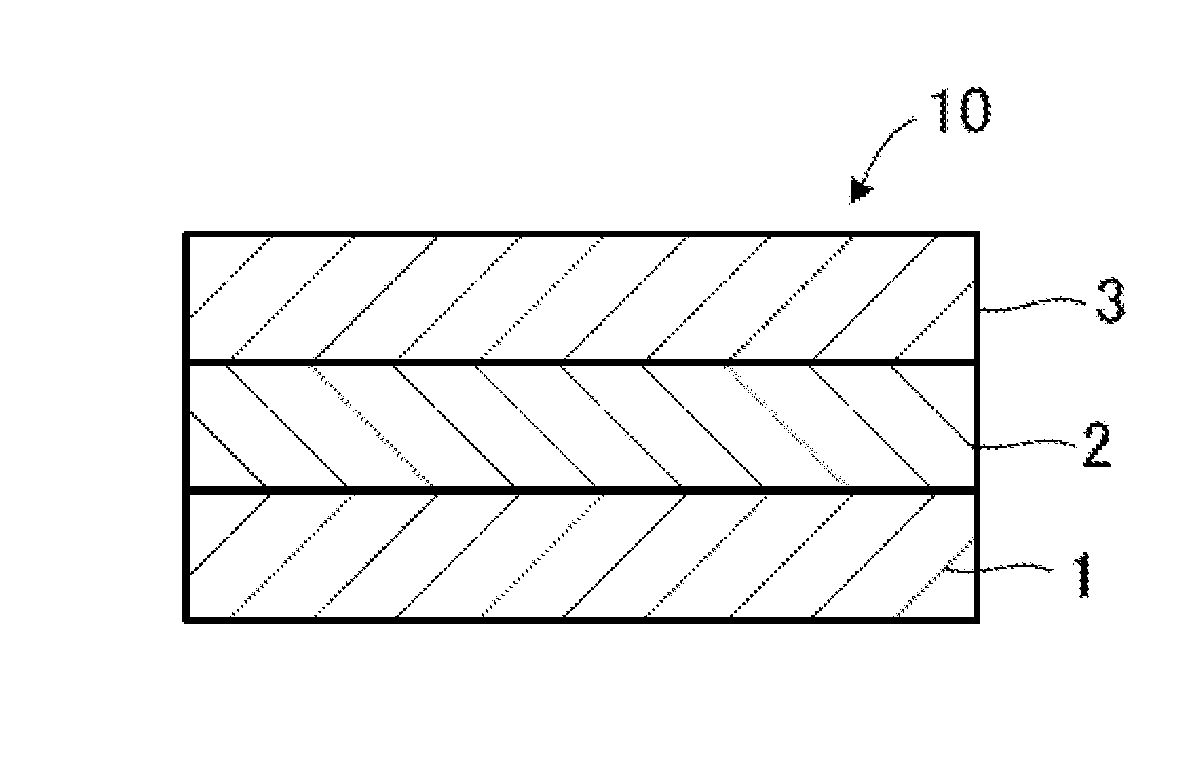
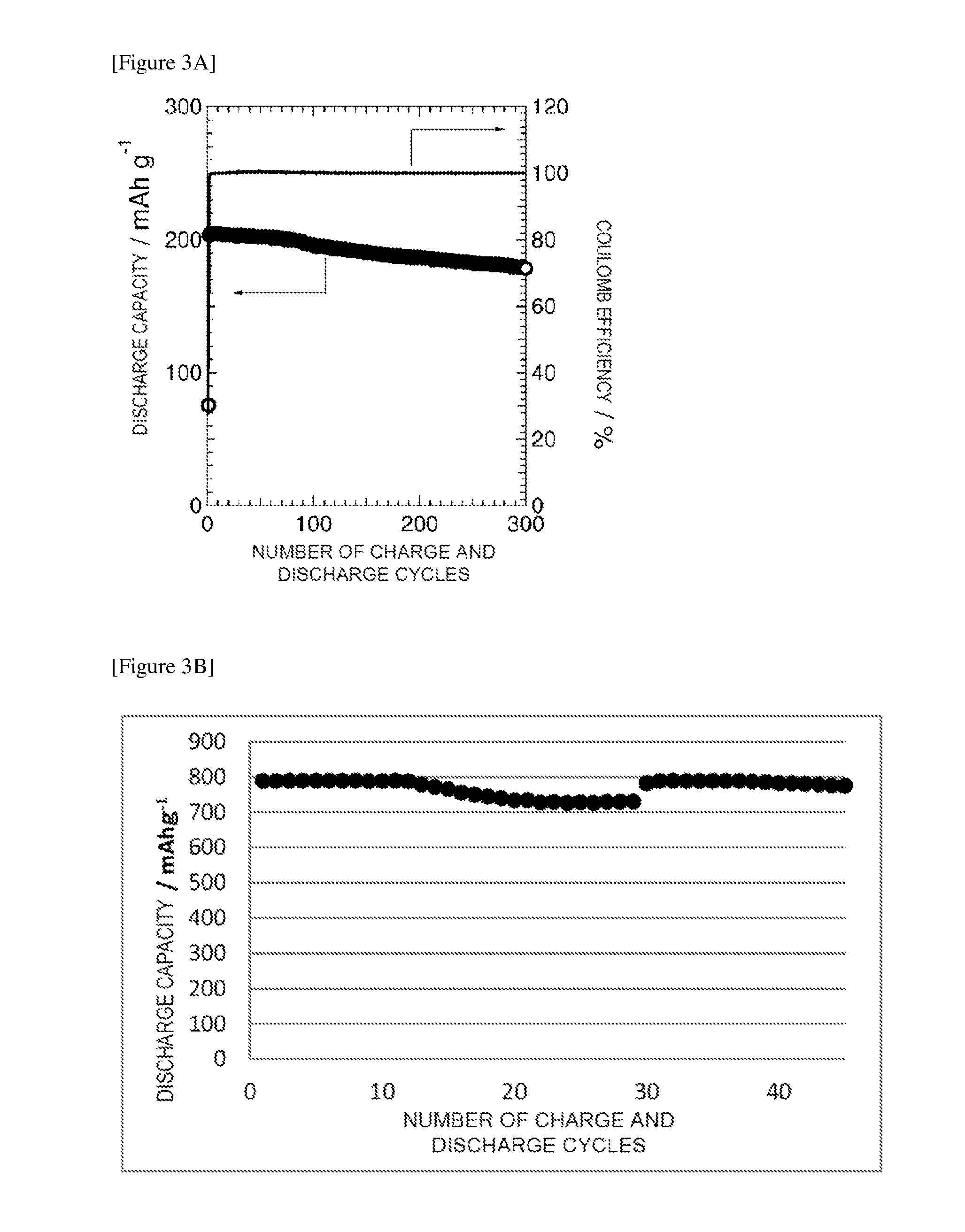
Patents
Literature
788 results about "Lithium ion conduction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
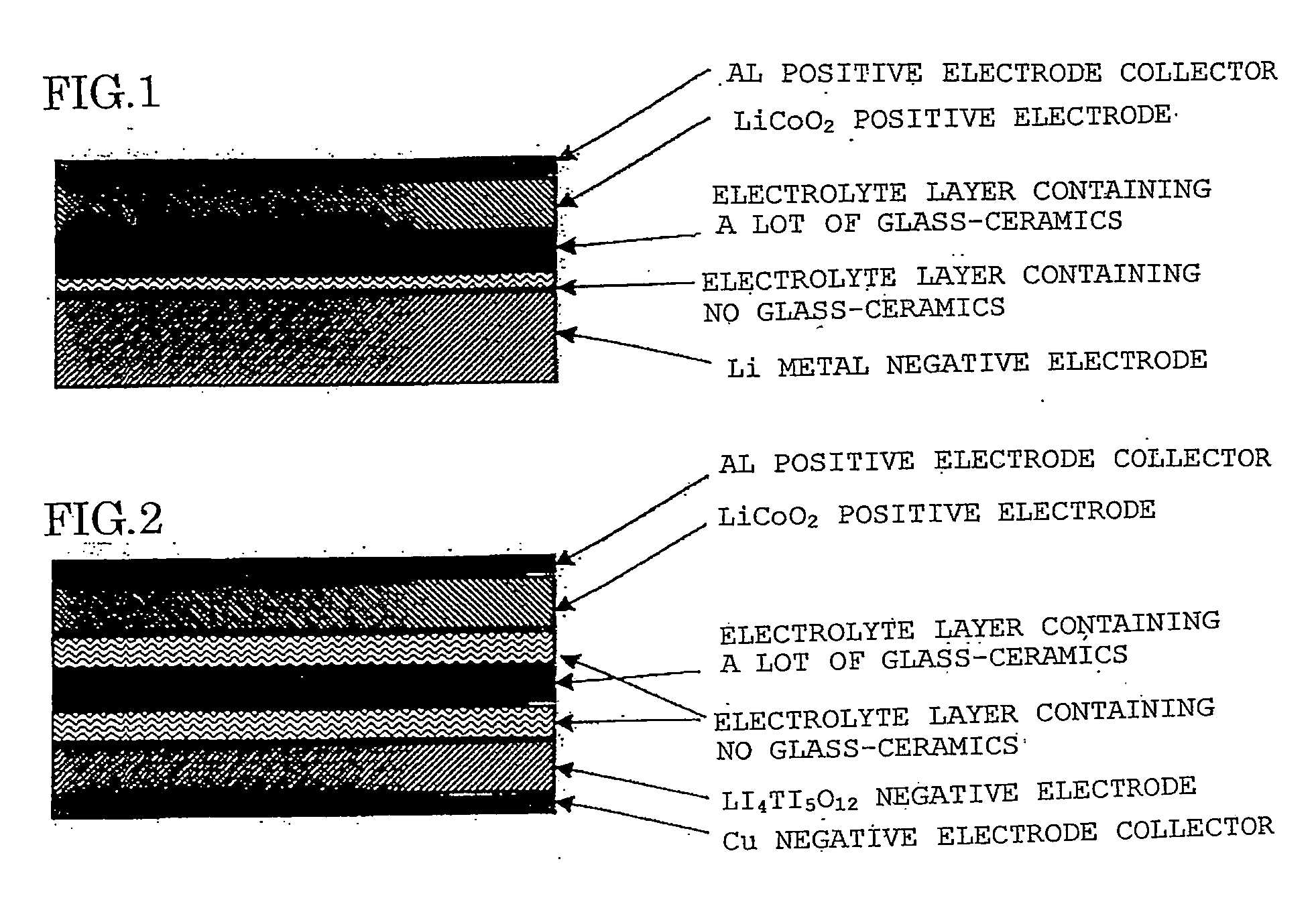
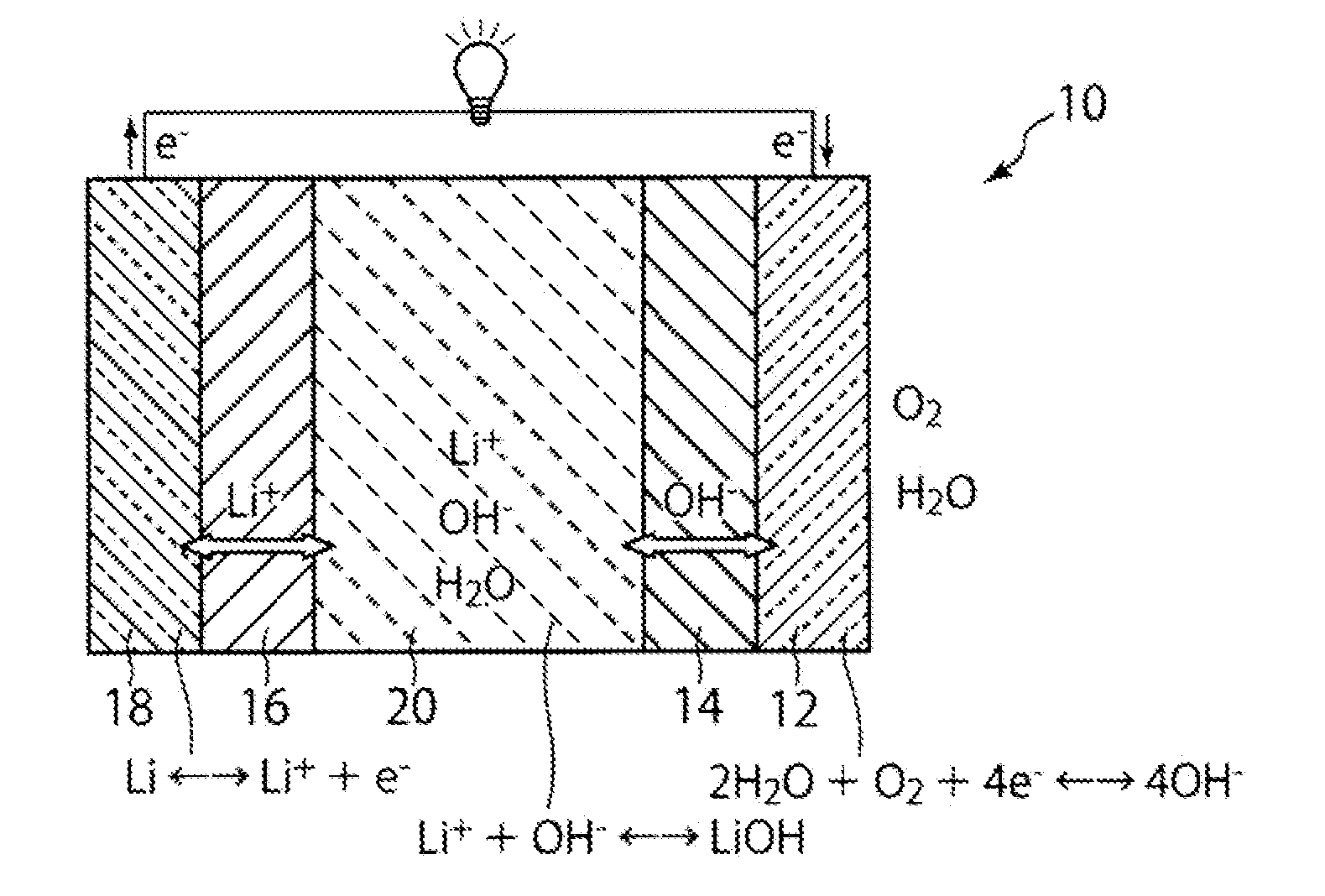
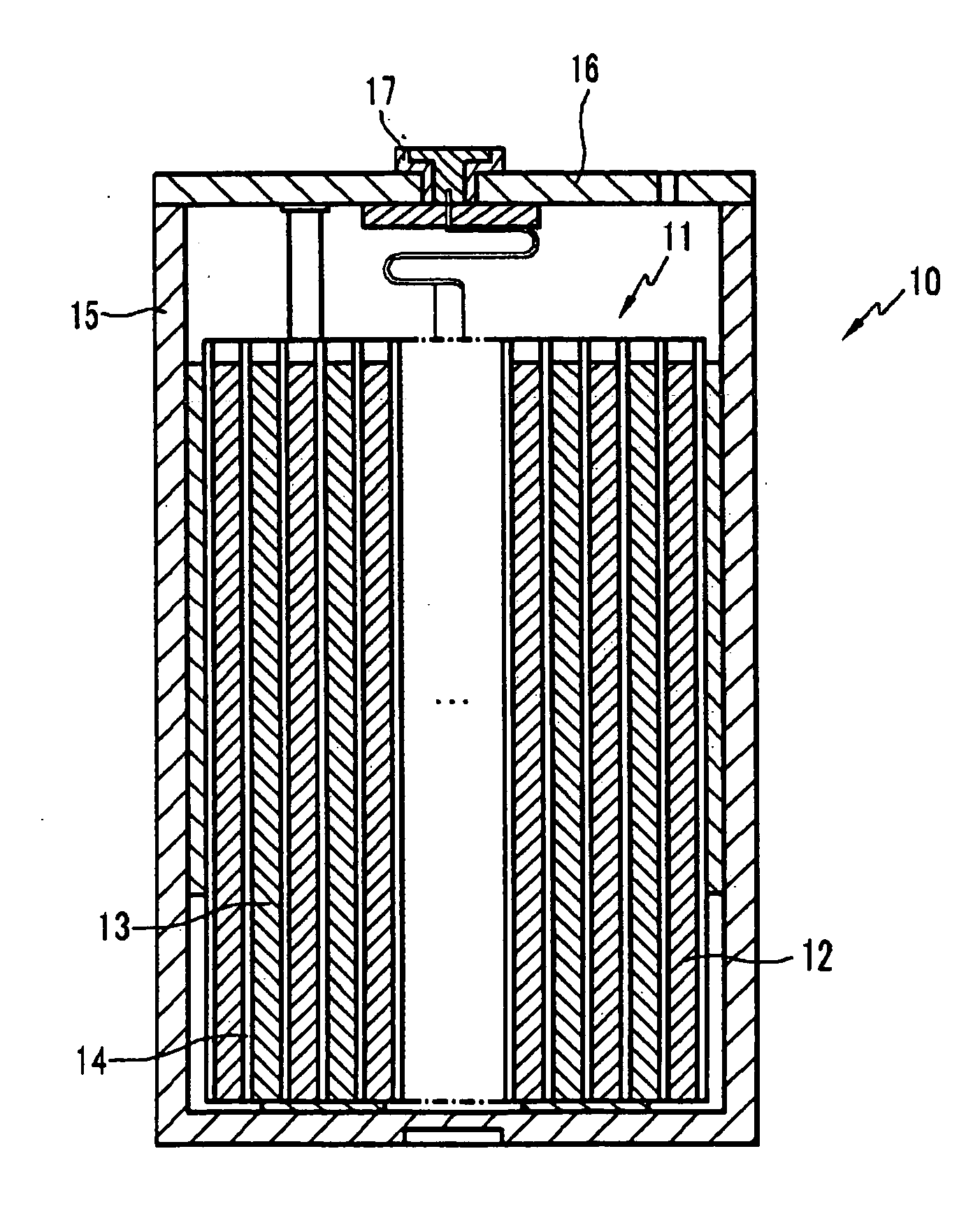
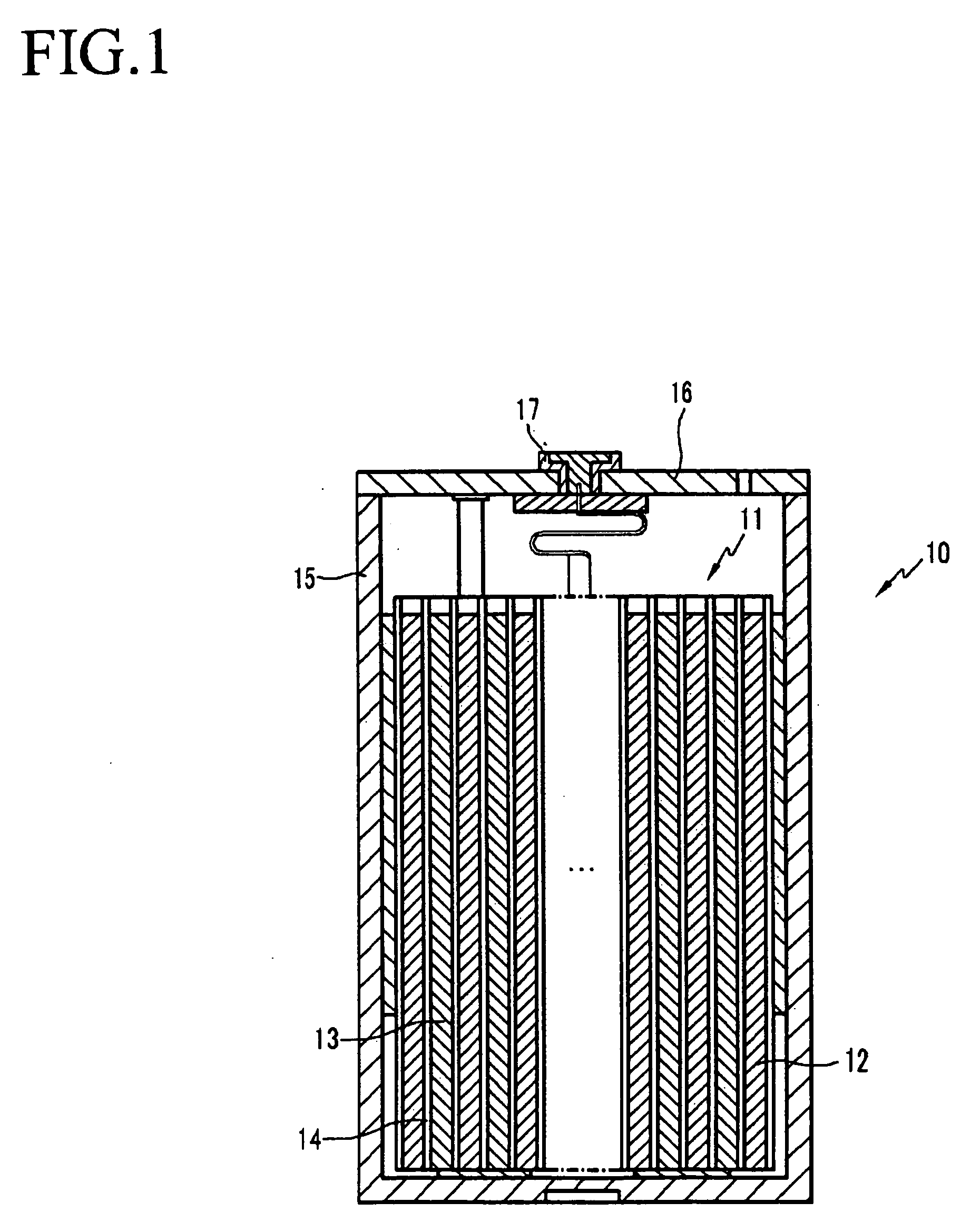
All-solid lithium battery
ActiveUS20090081554A1Improve output performanceLayer formationSolid electrolytesElectrode carriers/collectorsRedoxSulfide
Owner:NAT INST FOR MATERIALS SCI
Secondary lithium ion battery containing a prelithiated anode
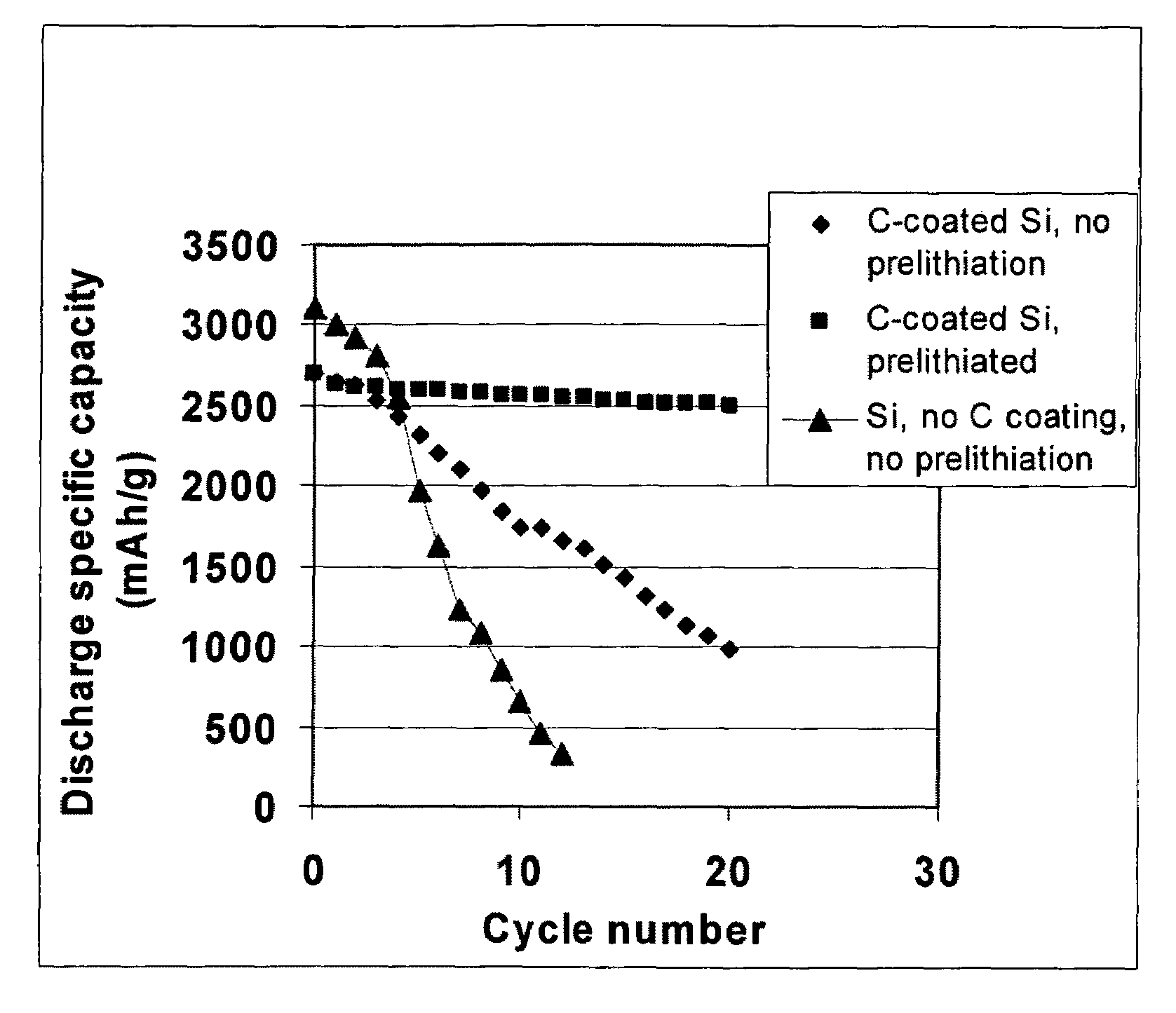
ActiveUS20100173198A1High specific capacityLong charge-discharge cycle lifeSecondary cellsActive material electrodesCharge dischargeGraphene
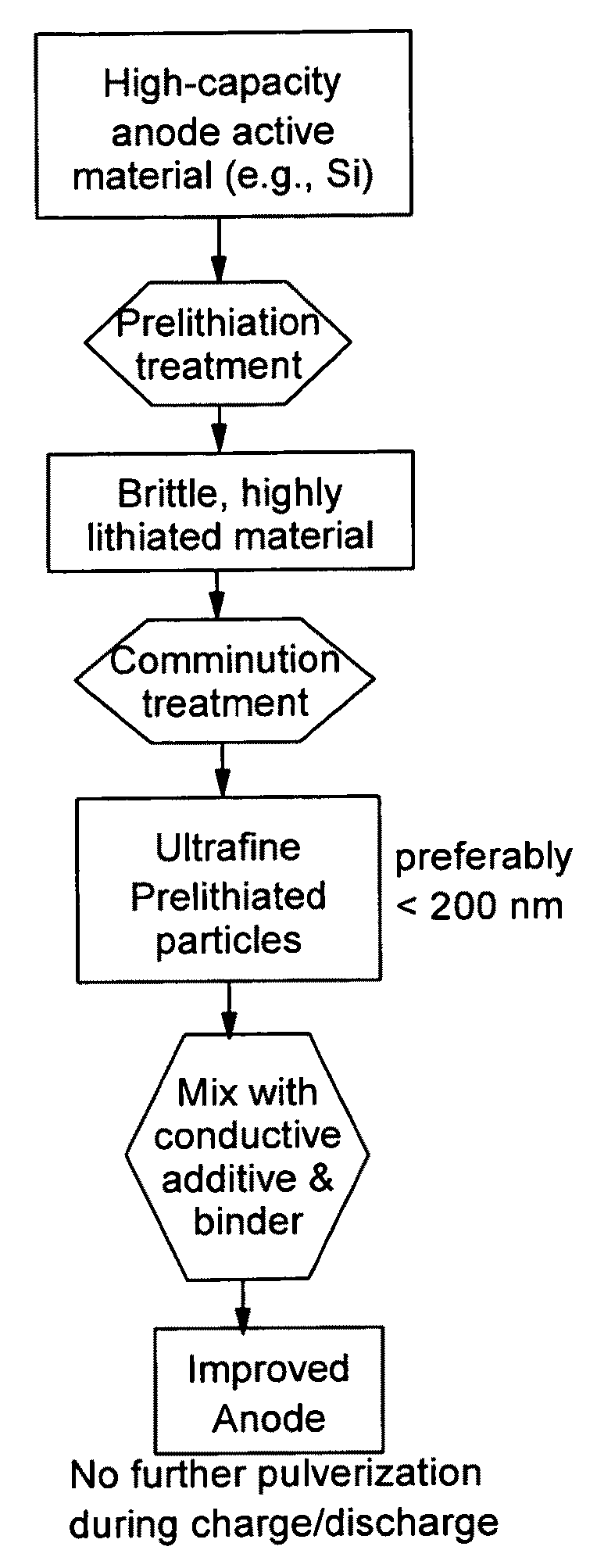
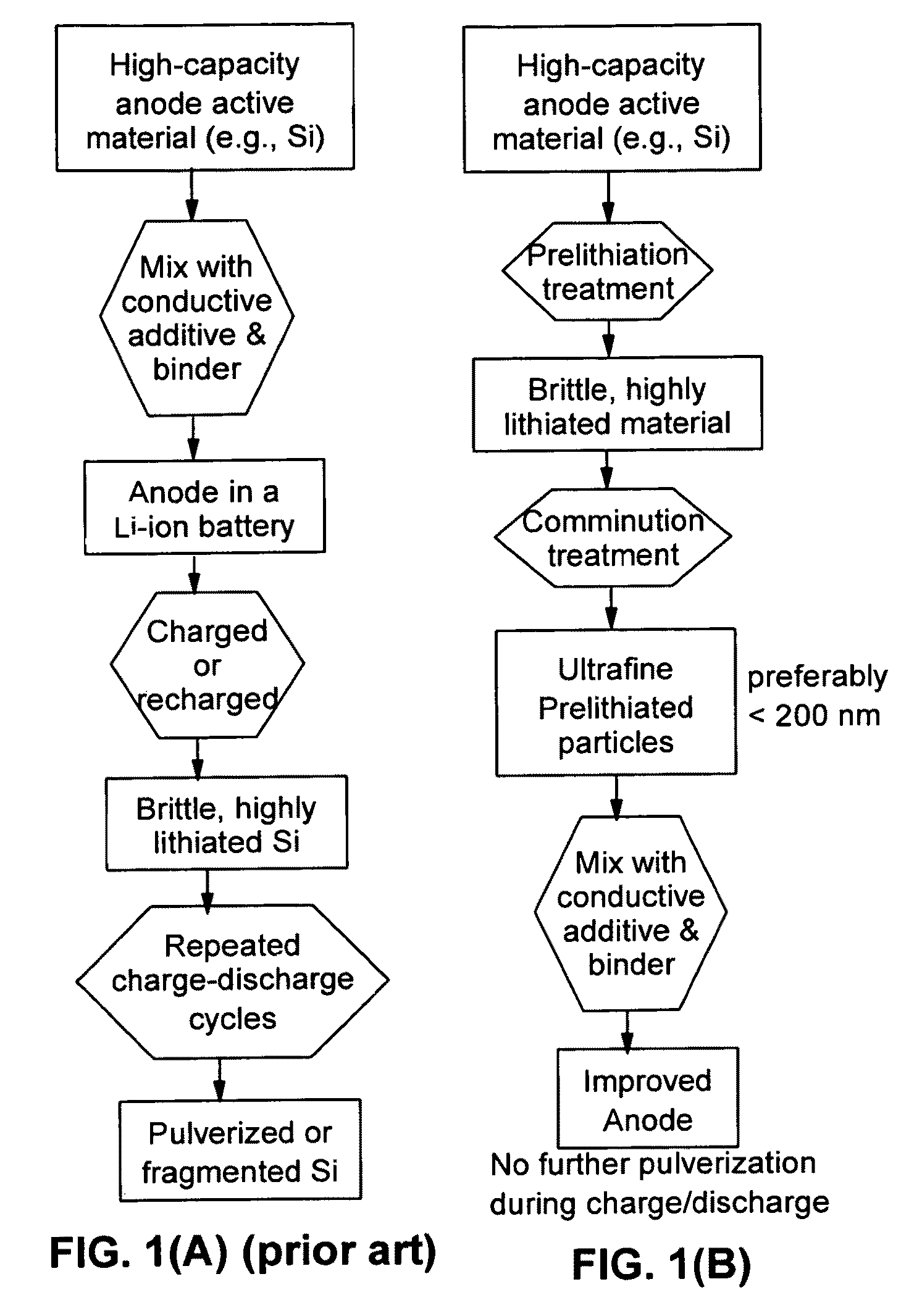
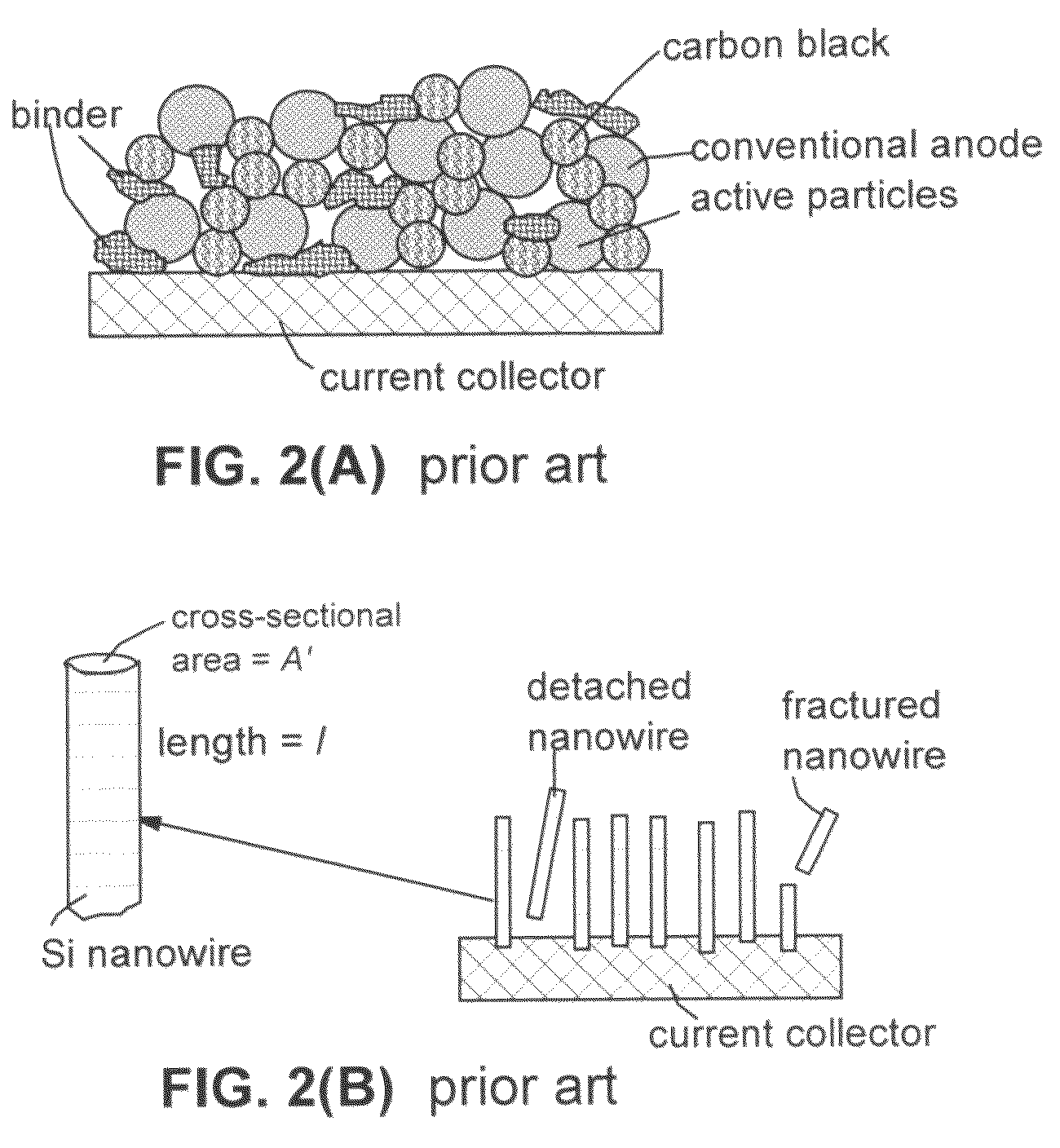
The present invention provides a lithium ion battery that exhibits a significantly improved specific capacity and much longer charge-discharge cycle life. In one preferred embodiment, the battery comprises an anode active material that has been prelithiated and pre-pulverized. This anode may be prepared with a method that comprises (a) providing an anode active material (preferably in the form of fine powder or thin film); (b) intercalating or absorbing a desired amount of lithium into the anode active material to produce a prelithiated anode active material; (c) comminuting the prelithiated anode active material into fine particles with an average size less than 10 μm (preferably <1 μm and most preferably <200 nm); and (d) combining multiple fine particles of the prelithiated anode active material with a conductive additive and / or a binder material to form the anode. Preferably, the prelithiated particles are protected by a lithium ion-conducting matrix or coating material. Further preferably, the matrix material is reinforced with nano graphene platelets.
Owner:GLOBAL GRAPHENE GRP INC
Lithium ion conductive solid electrolyte and production process thereof
InactiveUS20070231704A1Increase battery capacitySimple and convenient manufactureSecondary cellsSolid electrolyte cellsPorosityLithium metal
A lithium ion conductive solid electrolyte formed by sintering a molding product containing an inorganic powder and having a porosity of 10 vol % or less, which is obtained by preparing a molding product comprising an inorganic powder as a main ingredient and sintering the molding product after pressing and / or sintering the same while pressing, the lithium ion conductive solid electrolyte providing a solid electrolyte having high battery capacity without using a liquid electrolyte, usable stably for a long time and simple and convenient in manufacture and handling also in industrial manufacture in the application use of secondary lithium ion battery or primary lithium battery, a solid electrolyte having good charge / discharge cyclic characteristic in the application use of the secondary lithium ion battery a solid electrolyte with less water permeation and being safe when used for lithium metal-air battery in the application use of primary lithium battery, a manufacturing method of the solid electrolyte, and a secondary lithium ion battery and a primary lithium battery using the solid electrolyte.
Owner:OHARA
Garnet-type lithium ion-conducting oxide and all-solid-state lithium ion secondary battery containing the same
ActiveUS20110244337A1Improve lithium ion conductivitySmall rate of changeSolid electrolyte cellsElectrolytesAll solid stateIntensity normalization
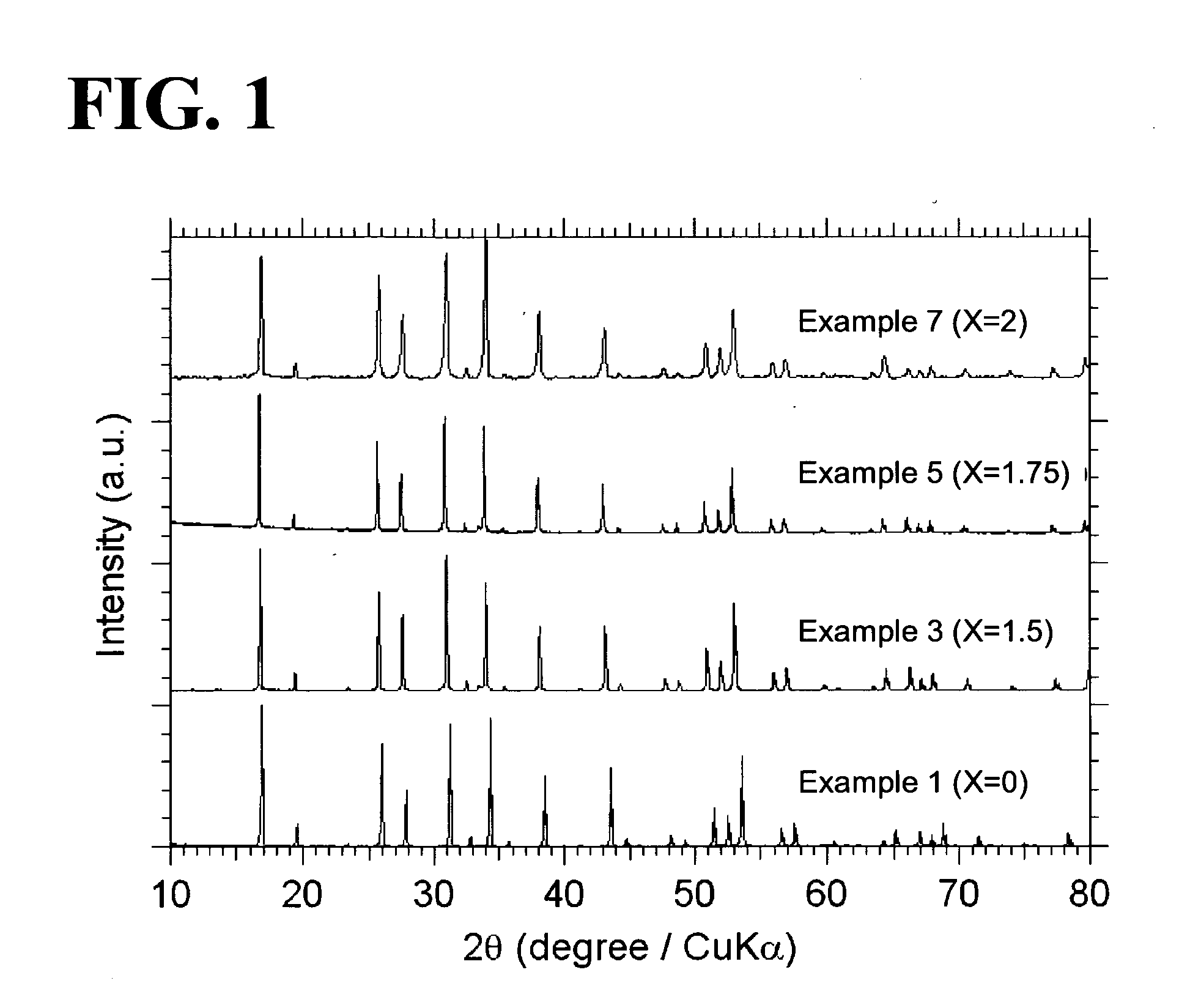
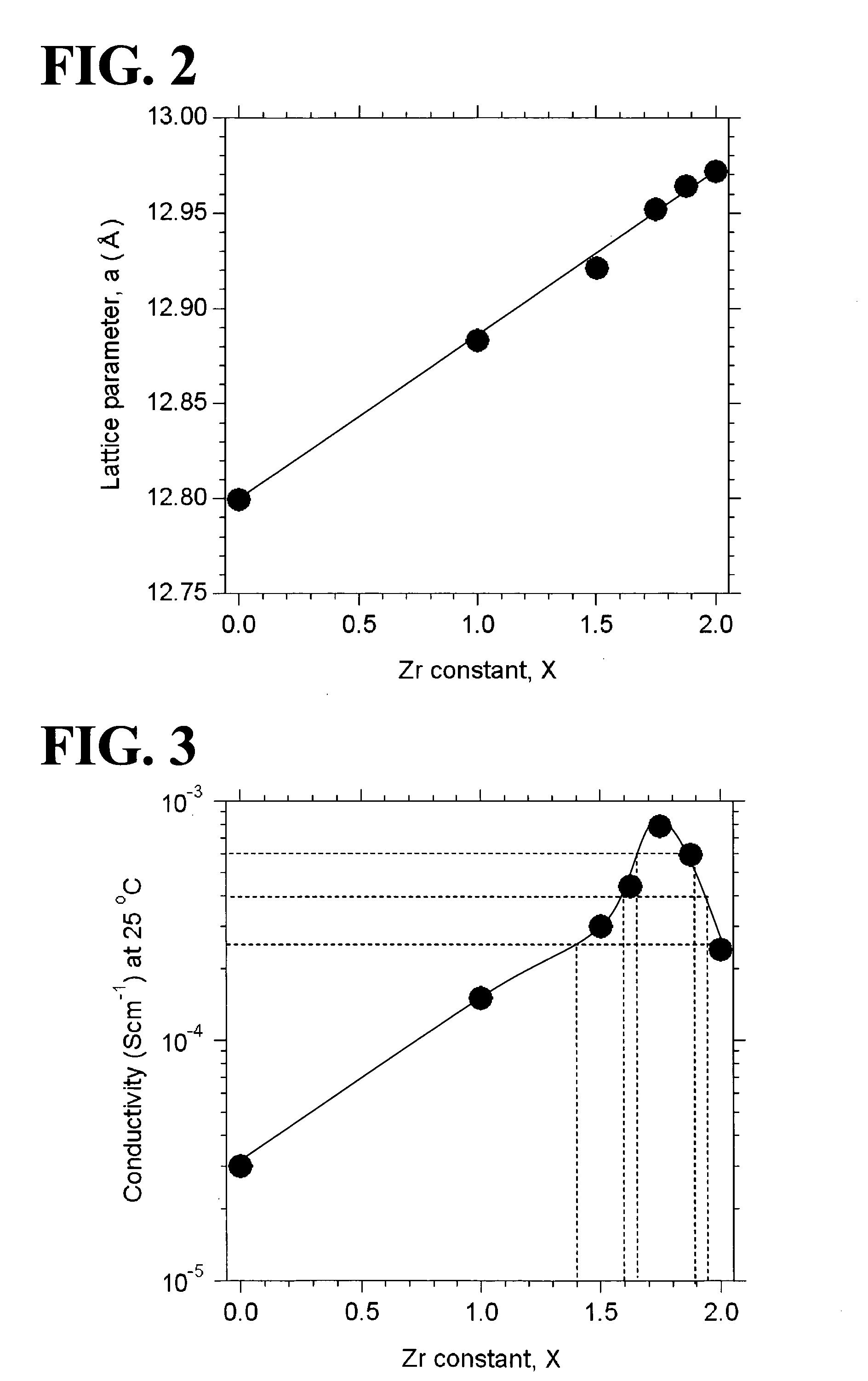
An all-solid-state lithium ion secondary battery containing a novel garnet-type oxide serving as a solid electrolyte. The garnet-type lithium ion-conducting oxide is one represented by the formula Li5+XLa3(ZrX, A2-X)O12, wherein A is at least one selected from the group consisting of Sc, Ti, V, Y, Nb, Hf, Ta, Al, Si, Ga, Ge, and Sn and X satisfies the inequality 1.4≦X<2, or is one obtained by substituting an element having an ionic radius different from that of Zr for Zr sites in an garnet-type lithium ion-conducting oxide represented by the formula Li7La3Zr2O12, wherein the normalized intensity of an X-ray diffraction (XRD) pattern with a diffraction peak, as normalized on the basis of the intensity of a diffraction peak, is 9.2 or more.
Owner:TOYOTA CENT RES & DEV LAB INC
Lithium ion conductive solid electrolyte and method for manufacturing the same
ActiveUS20070087269A1Increase battery capacityImprove discharge characteristicsSolid electrolytesPhosphatesLithiumPorosity
Owner:OHARA
Secondary lithium ion battery containing a prelithiated anode
ActiveUS8241793B2Increase capacityQuick releaseActive material electrodesSecondary cellsHost materialCharge discharge
A lithium ion battery that exhibits a significantly improved specific capacity and much longer charge-discharge cycle life. The battery comprises an anode active material that has been prelithiated and pre-pulverized. This anode may be prepared with a method that comprises (a) providing an anode active material; (b) intercalating or absorbing a desired amount of lithium into the anode active material to produce a prelithiated anode active material; (c) comminuting the prelithiated anode active material into fine particles with an average size less than 10 μm (preferably <1 μm and most preferably <200 nm); and (d) combining multiple fine particles of the prelithiated anode active material with a conductive additive and / or a binder material to form the anode. Preferably, the prelithiated particles are protected by a lithium ion-conducting matrix or coating material. Further preferably, the matrix material is reinforced with nano graphene platelets.
Owner:GLOBAL GRAPHENE GRP INC
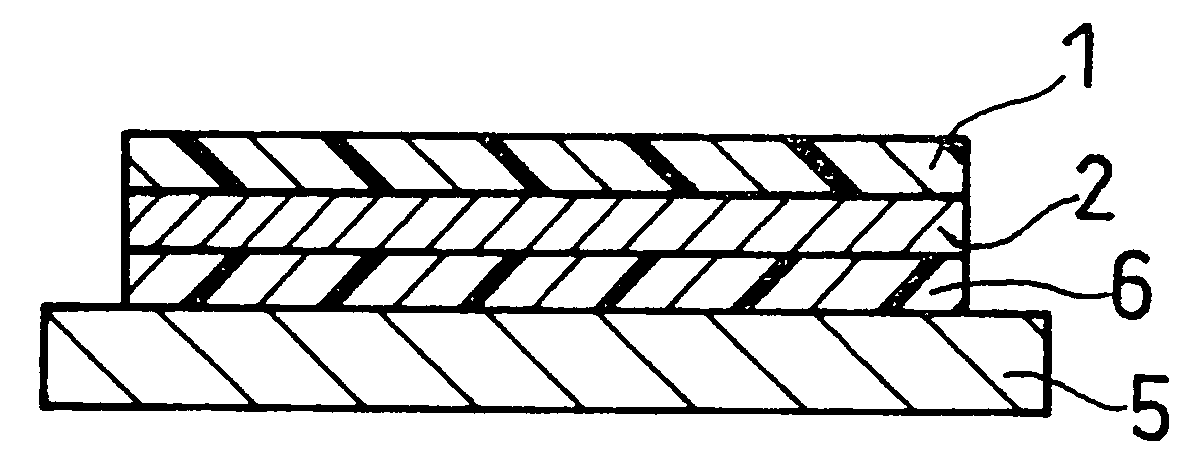
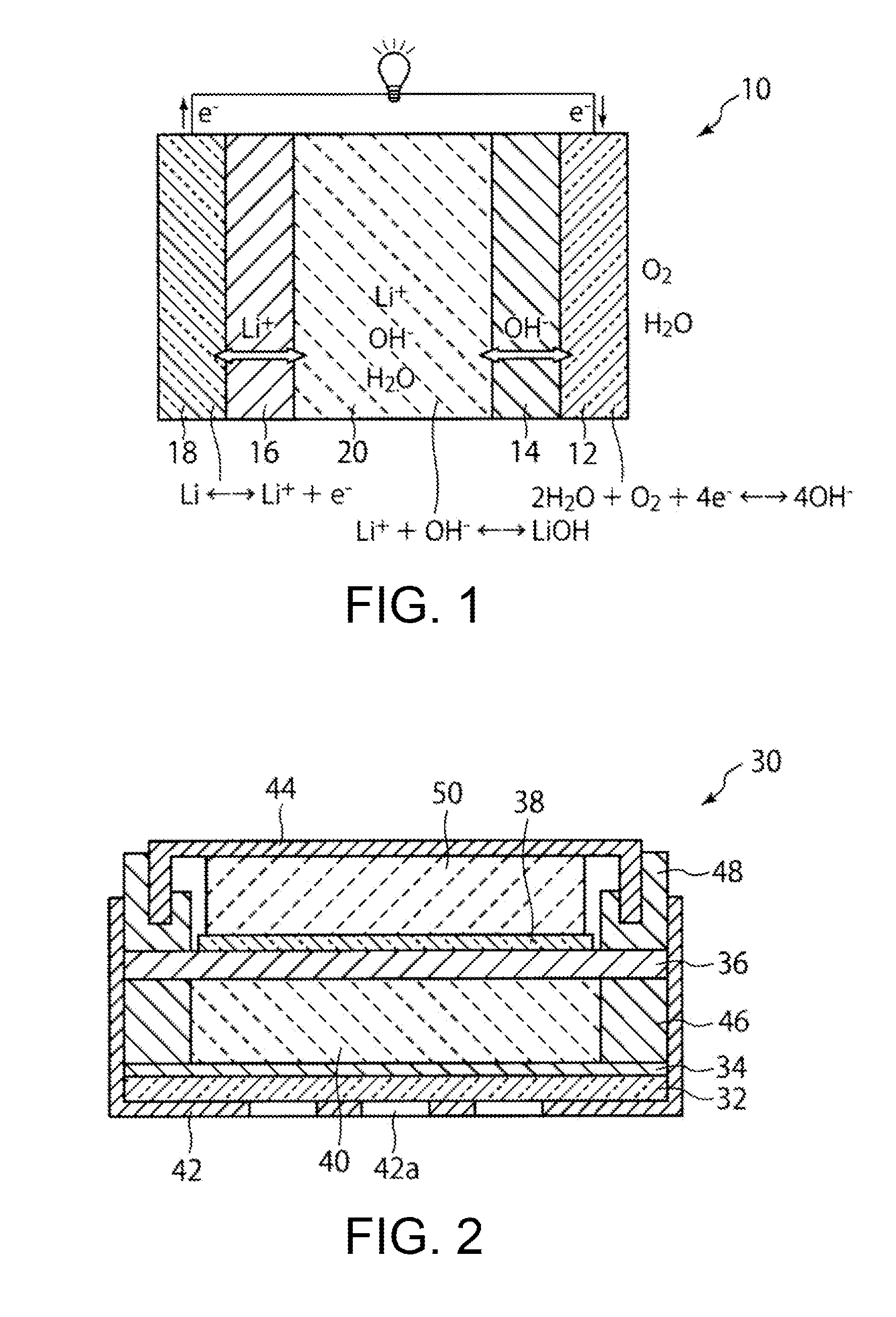
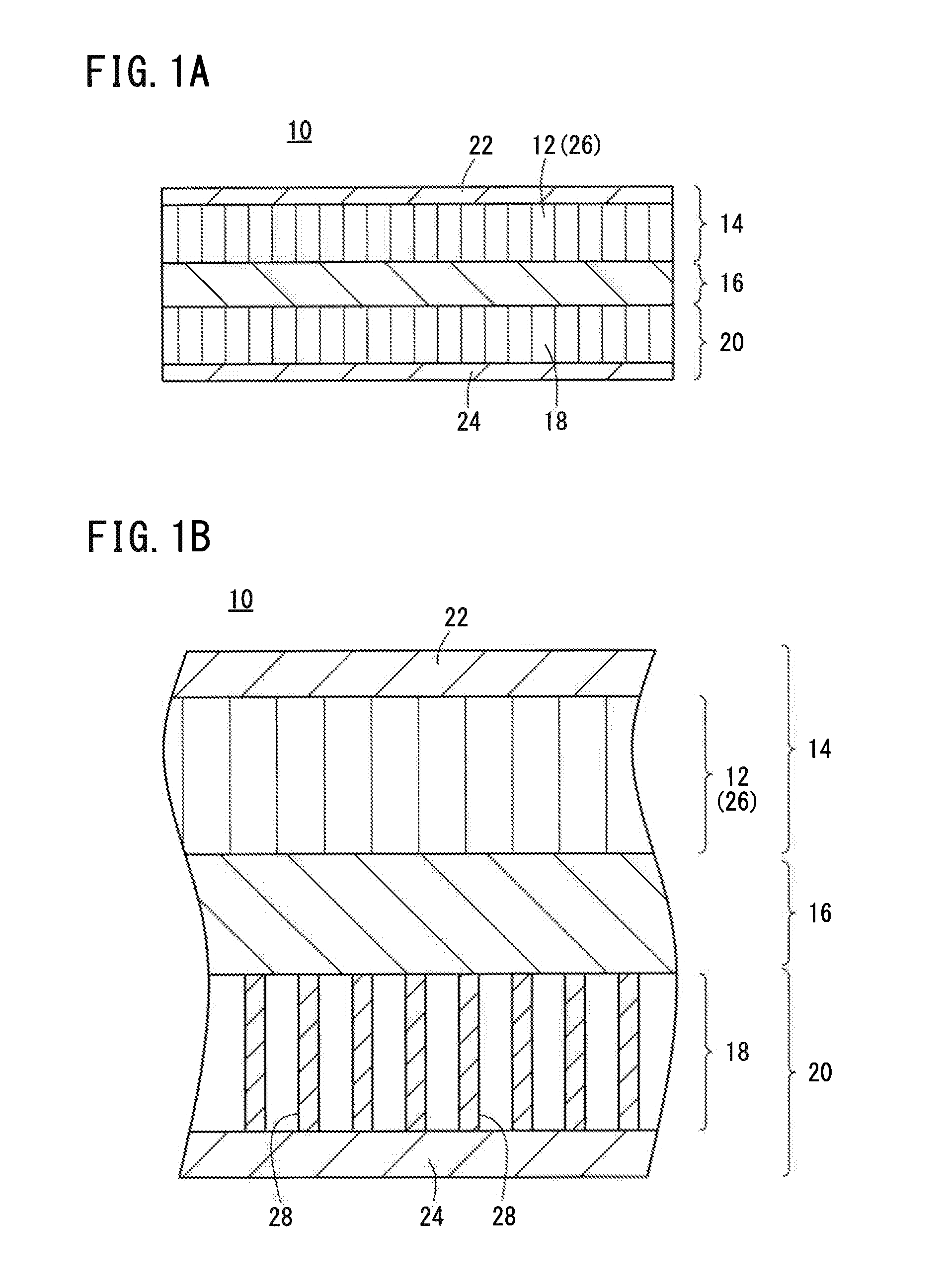
Laminate Including Active Material Layer and Solid Electrolyte Layer, and All Solid Lithium Secondary Battery Using the Same
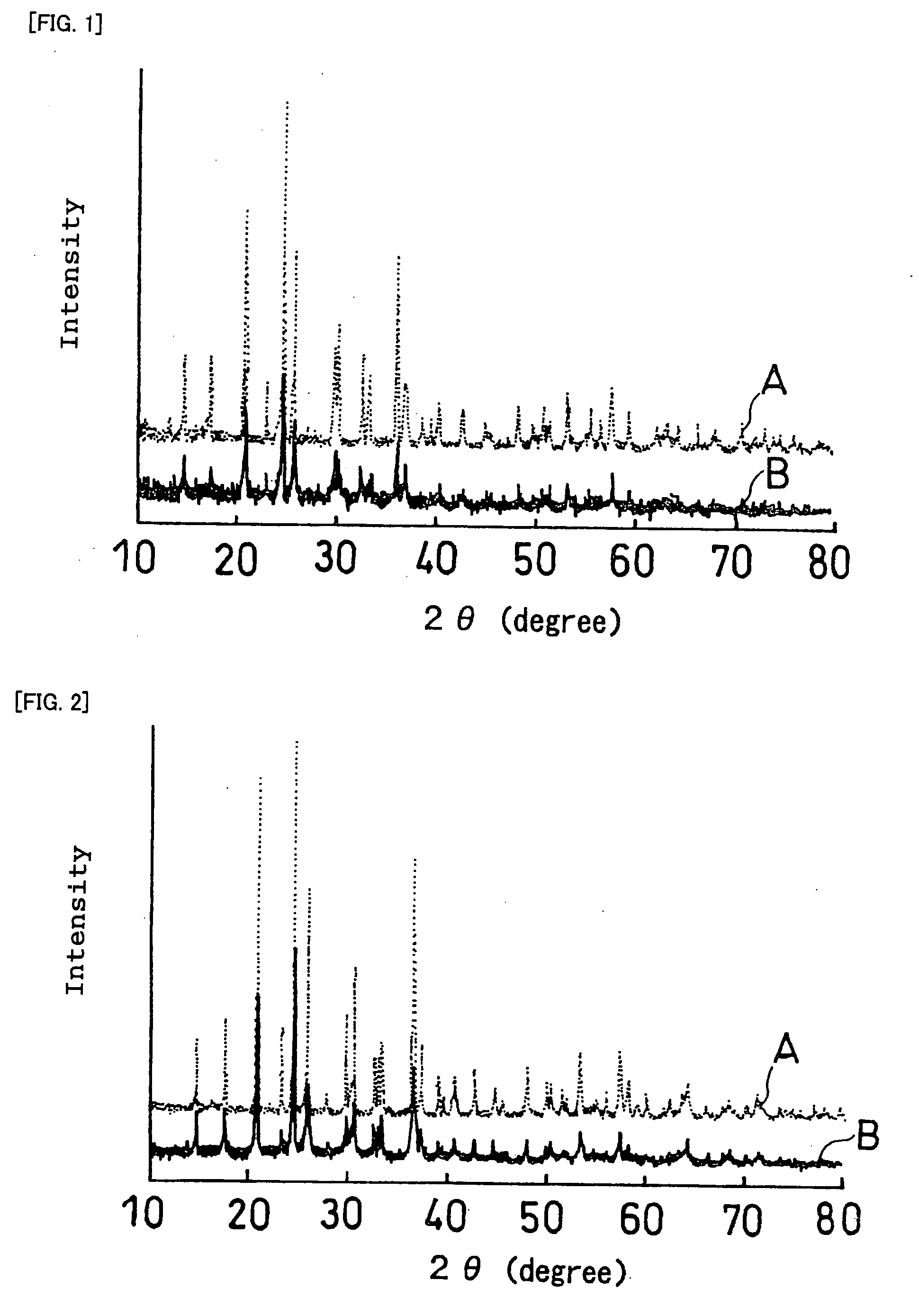
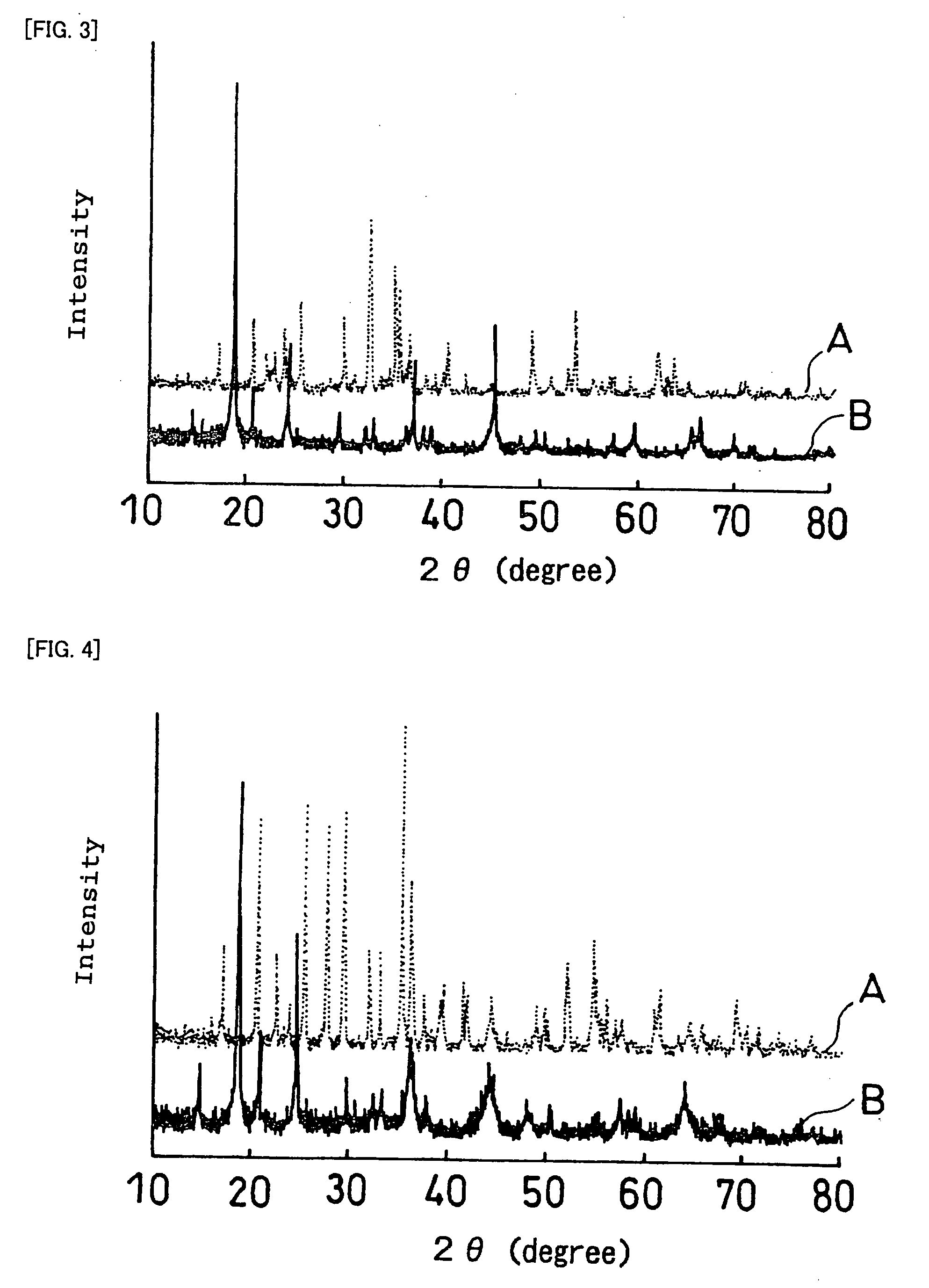
InactiveUS20070259271A1Improve life characteristicsSmall internal resistanceElectrode thermal treatmentFinal product manufactureLithiumX-ray
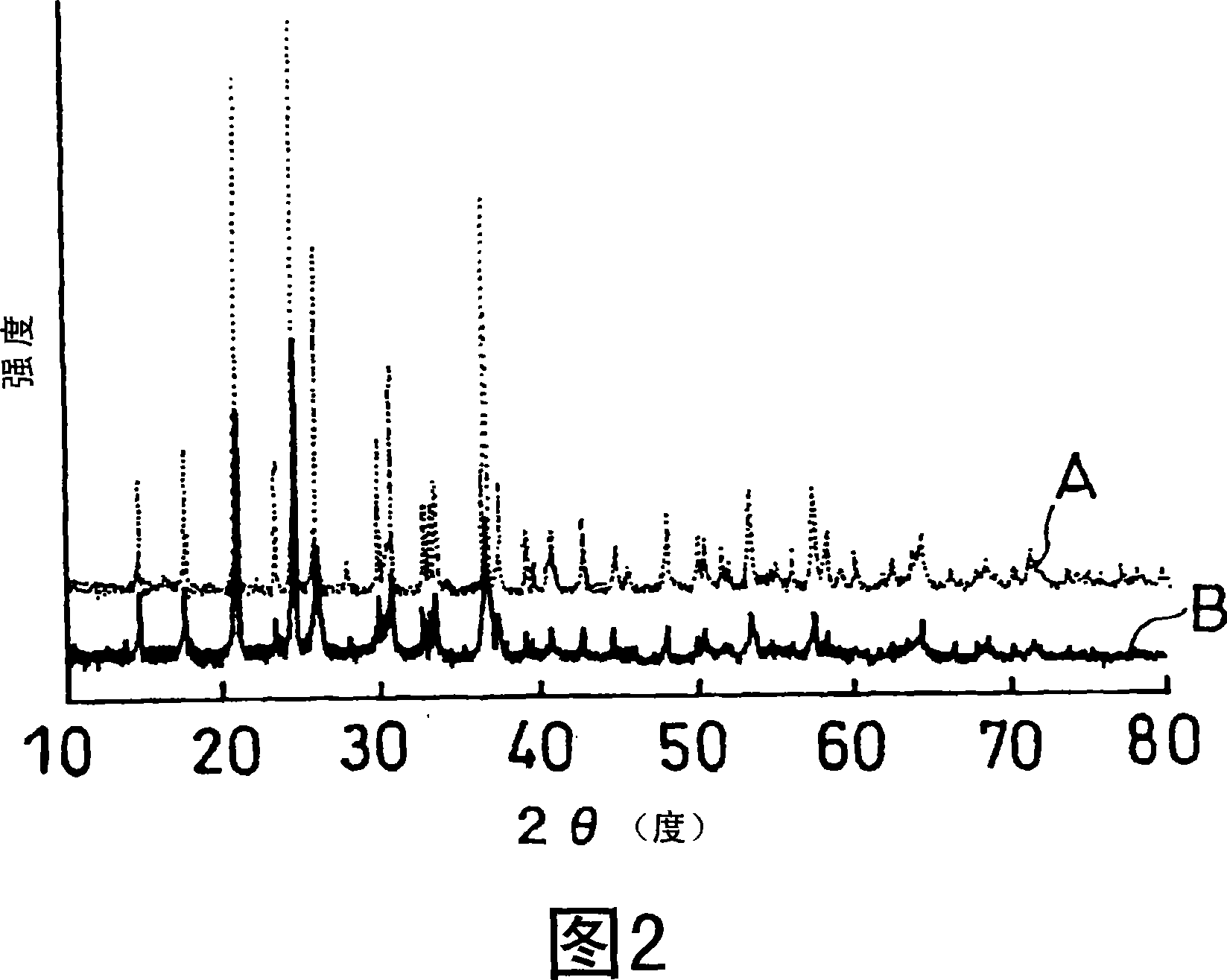
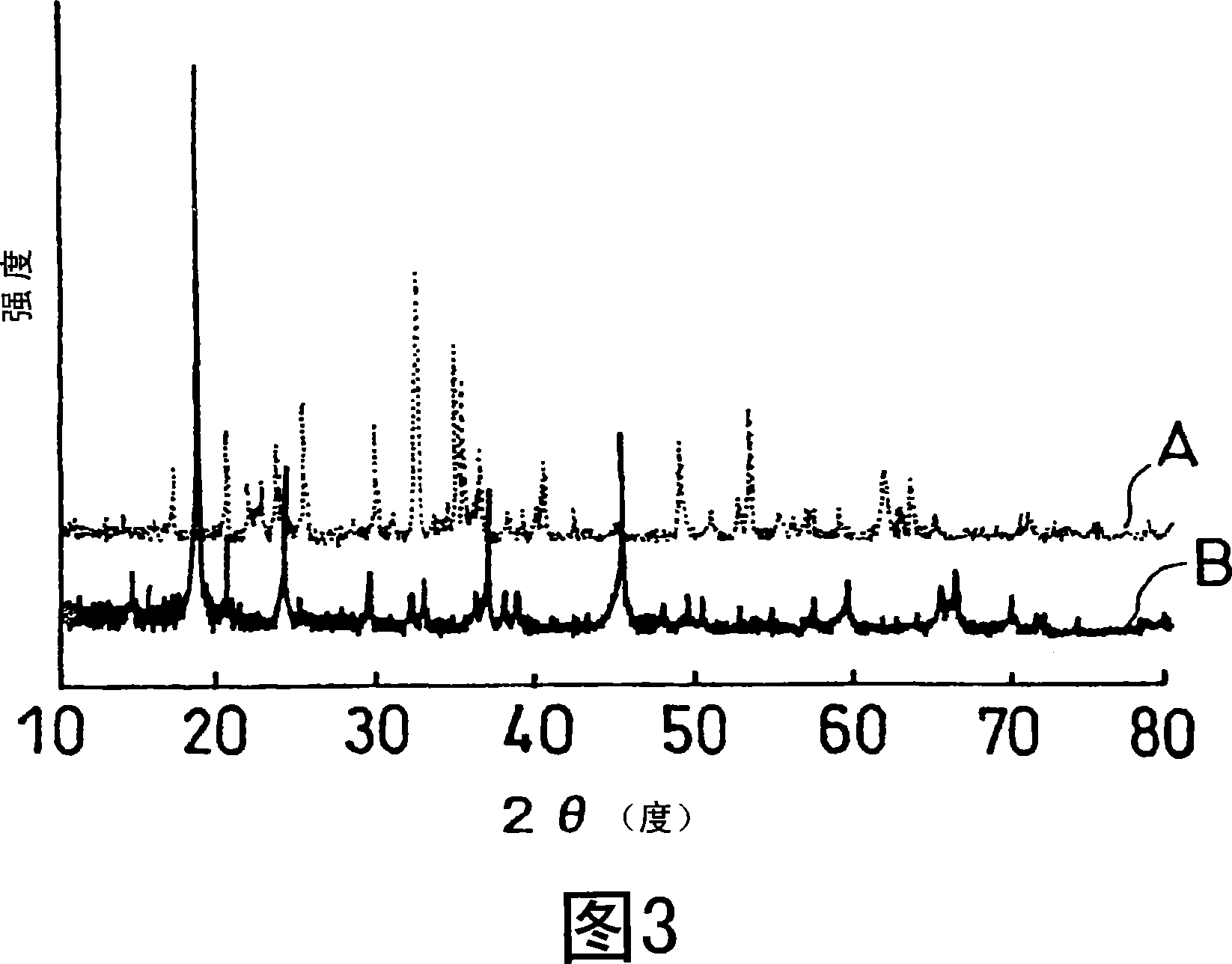
A laminate includes an active material layer and a solid electrolyte layer bonded to the active material layer by sintering. The active material layer includes a crystalline first substance capable of absorbing and desorbing lithium ions, and the solid electrolyte layer includes a crystalline second substance with lithium ion conductivity. An X-ray diffraction analysis of the laminate shows that there is no component other than constituent components of the active material layer and constituent components of the solid electrolyte layer. Also, an all solid lithium secondary battery includes such a laminate and a negative electrode active material layer.
Owner:PANASONIC CORP
Lithium ion conductive composite electrolyte and lithium ion secondary battery using same
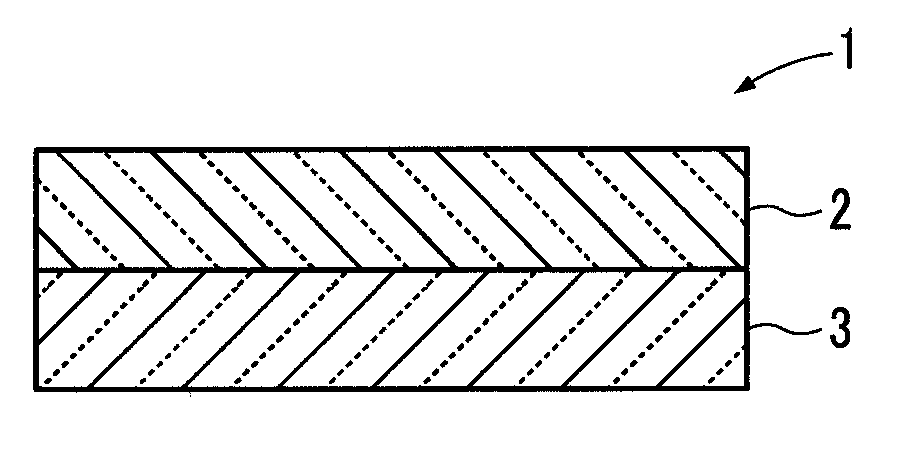
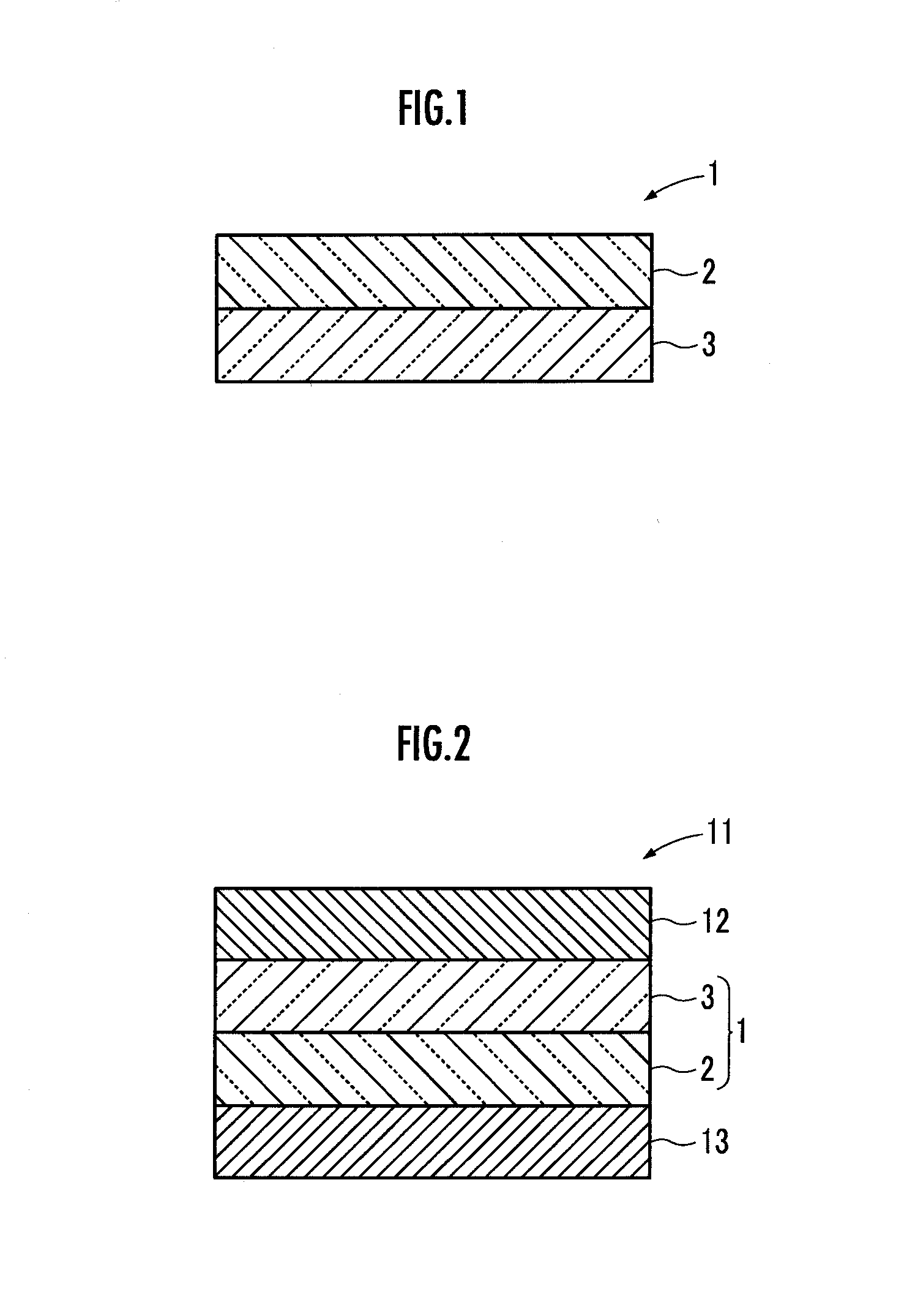
ActiveUS20130230778A1Improve cycle performancePrevent electrolyte leakageNon-aqueous electrolyte cellsSecondary cellsComposite electrolyteConductive polymer
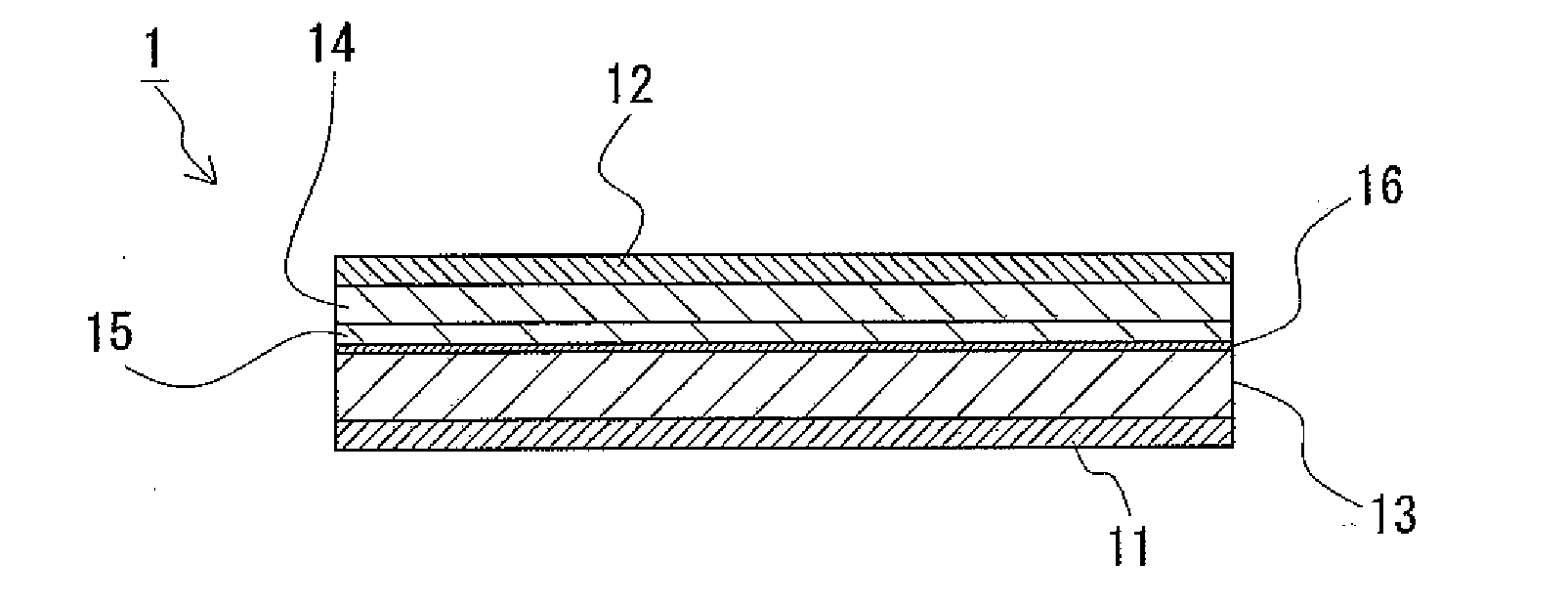
Provided is a lithium ion conductive composite electrolyte that can prevent leakage of electrolyte solution, and that have excellent cycle performance and lithium ion conductivity, and a lithium ion secondary battery. A lithium ion conductive composite electrolyte 1 is formed so that a lithium ion conductive polymer gel electrolyte is held in a porous body 2 formed from lithium ion conductive inorganic solid electrolyte particles and an organic polymer. The lithium ion conductive inorganic solid electrolyte particles are formed from a composite metal oxide that has a garnet structure, and is represented by the chemical formula Li7−yLa3−xAxZr2−yMyO12 (wherein 0≦x 3, 0≦y≦2, A is one of Y, Nd, Sm, and Gd, and M is Nb or Ta). A lithium ion secondary battery 11 includes the lithium ion conductive composite electrolyte 1 between a positive electrode 12 and a negative electrode 13.
Owner:HONDA MOTOR CO LTD
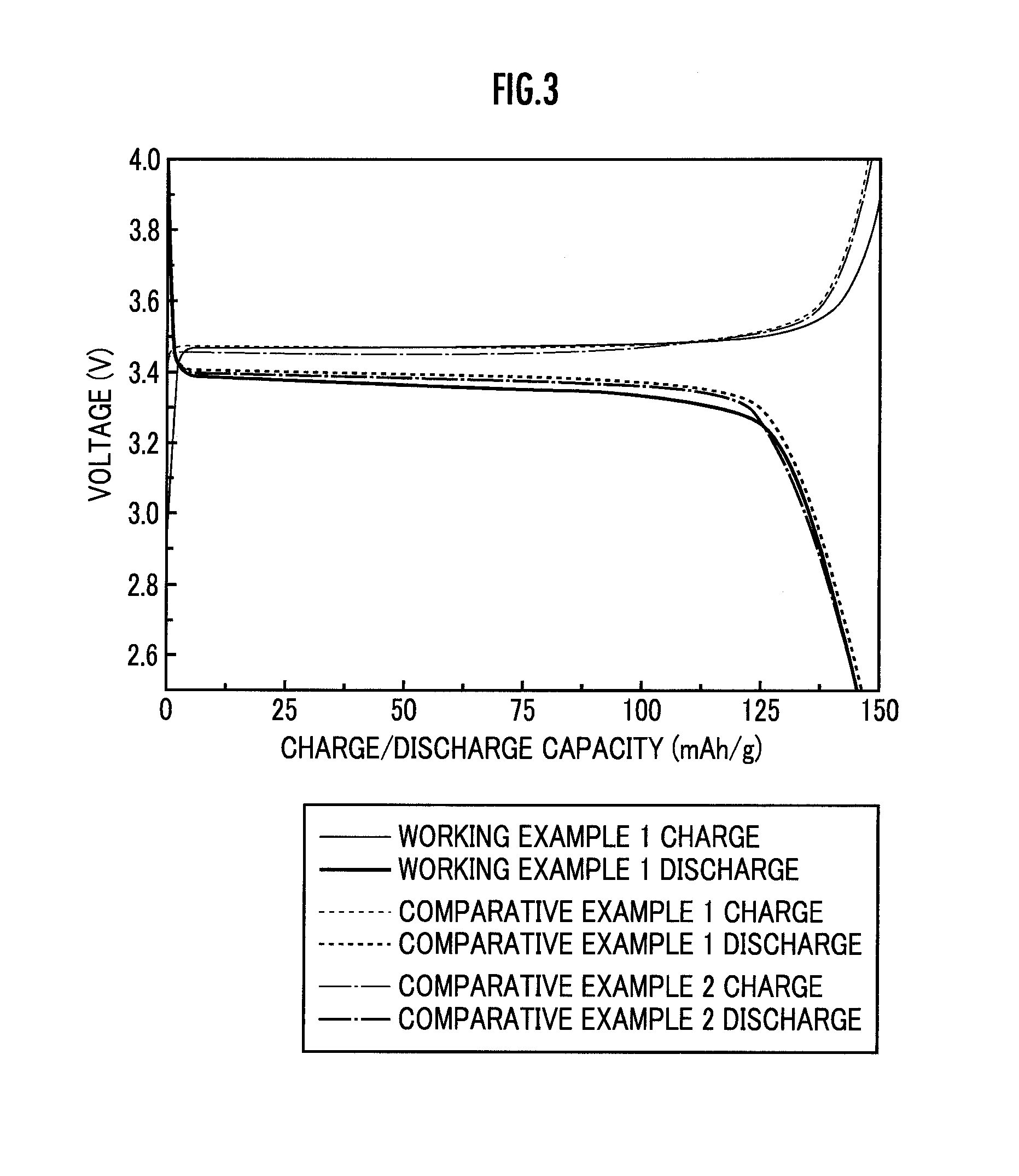
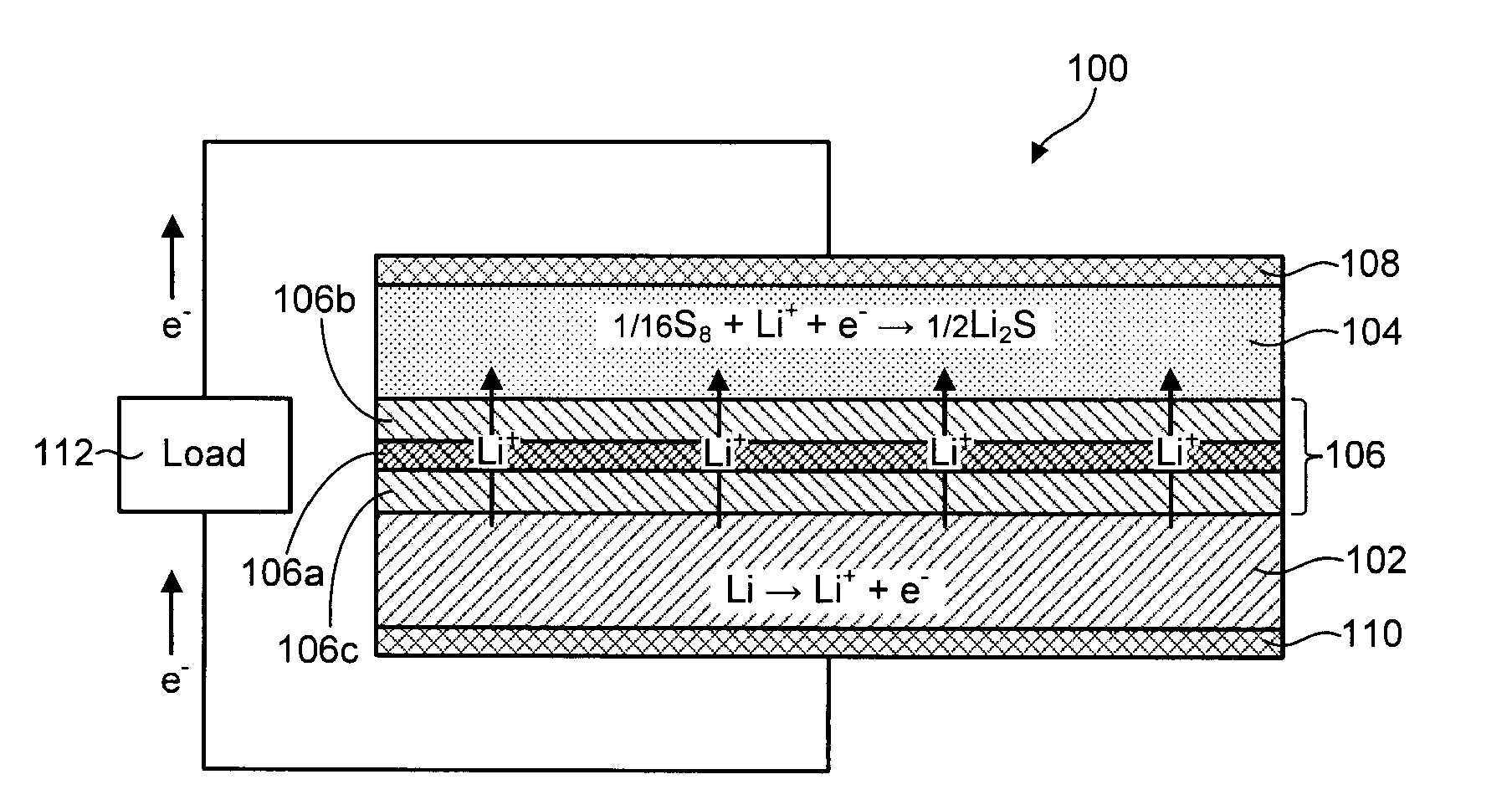
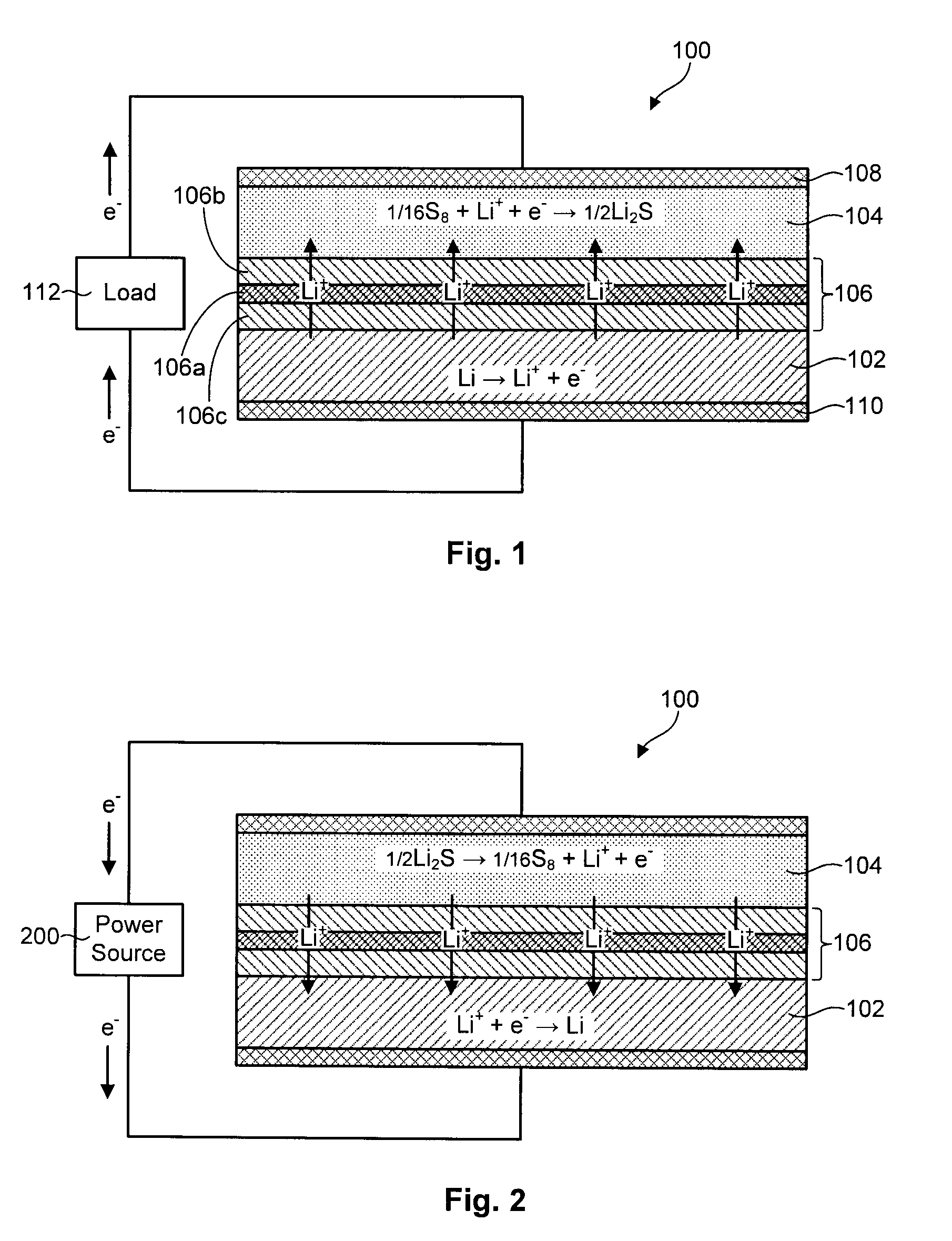
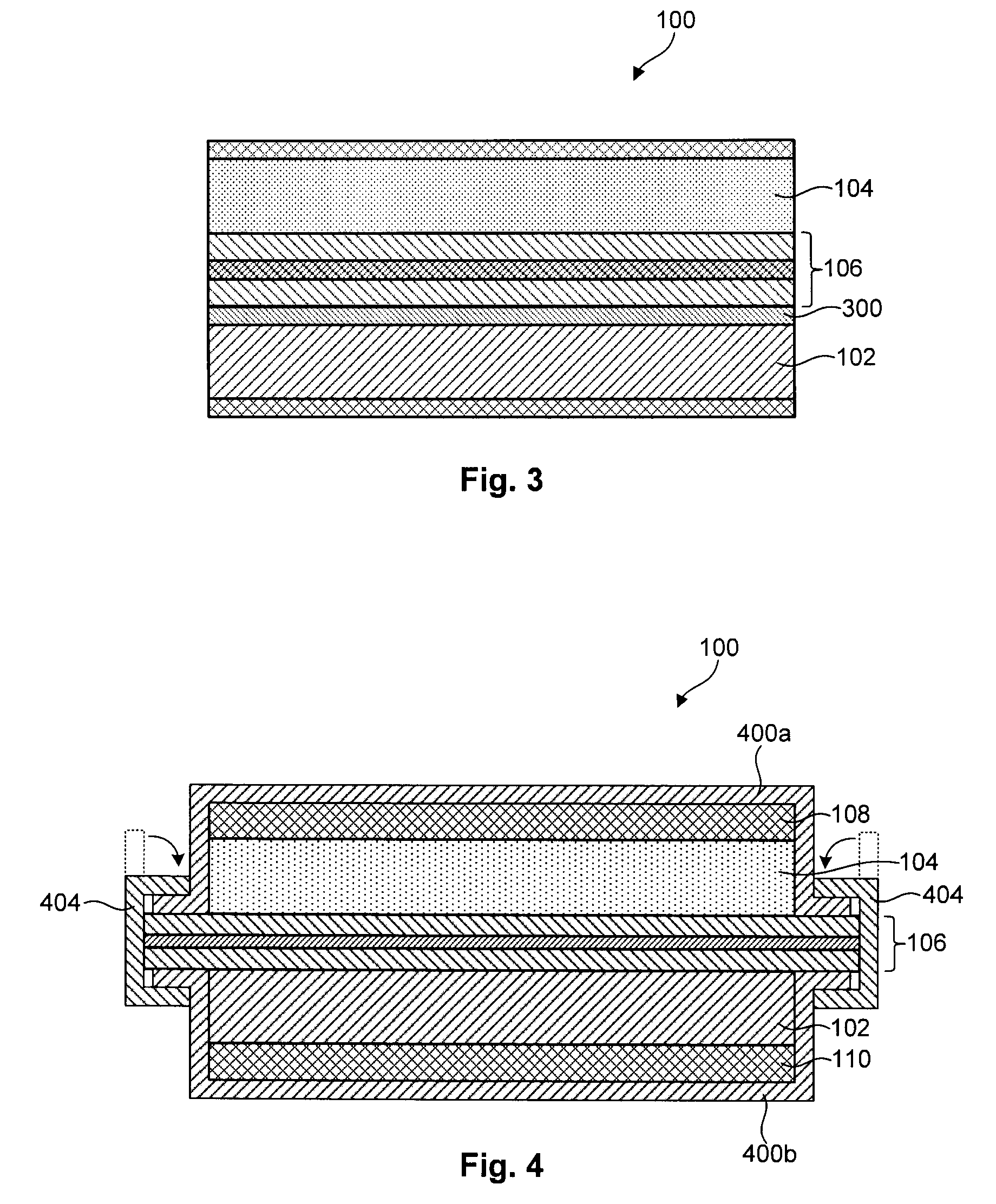
Lithium-sulfur battery with a substantially non-pourous membrane and enhanced cathode utilization
ActiveUS20090061288A1Avoid reactionOrganic electrolyte cellsSolid electrolyte cellsSulfurLithium–sulfur battery
A lithium-sulfur battery is disclosed in one embodiment of the invention as including an anode containing lithium and a cathode comprising elemental sulfur. The cathode may include at least one solvent selected to at least partially dissolve the elemental sulfur and Li2Sx. A substantially non-porous lithium-ion-conductive membrane is provided between the anode and the cathode to keep sulfur or other reactive species from migrating therebetween. In certain embodiments, the lithium-sulfur battery may include a separator between the anode and the non-porous lithium-ion-conductive membrane. This separator may prevent the lithium in the anode from reacting with the non-porous lithium-ion-conductive membrane. In certain embodiments, the separator is a porous separator infiltrated with a lithium-ion-conductive electrolyte.
Owner:ENLIGHTEN INNOVATIONS INC
Alkali ion conductive glass-ceramics and electric cells and gas sensors using the same
InactiveUS7211532B2Improve performanceImprove conductivityMaterial analysis by electric/magnetic meansSecondary cellsLithiumAlkali ions
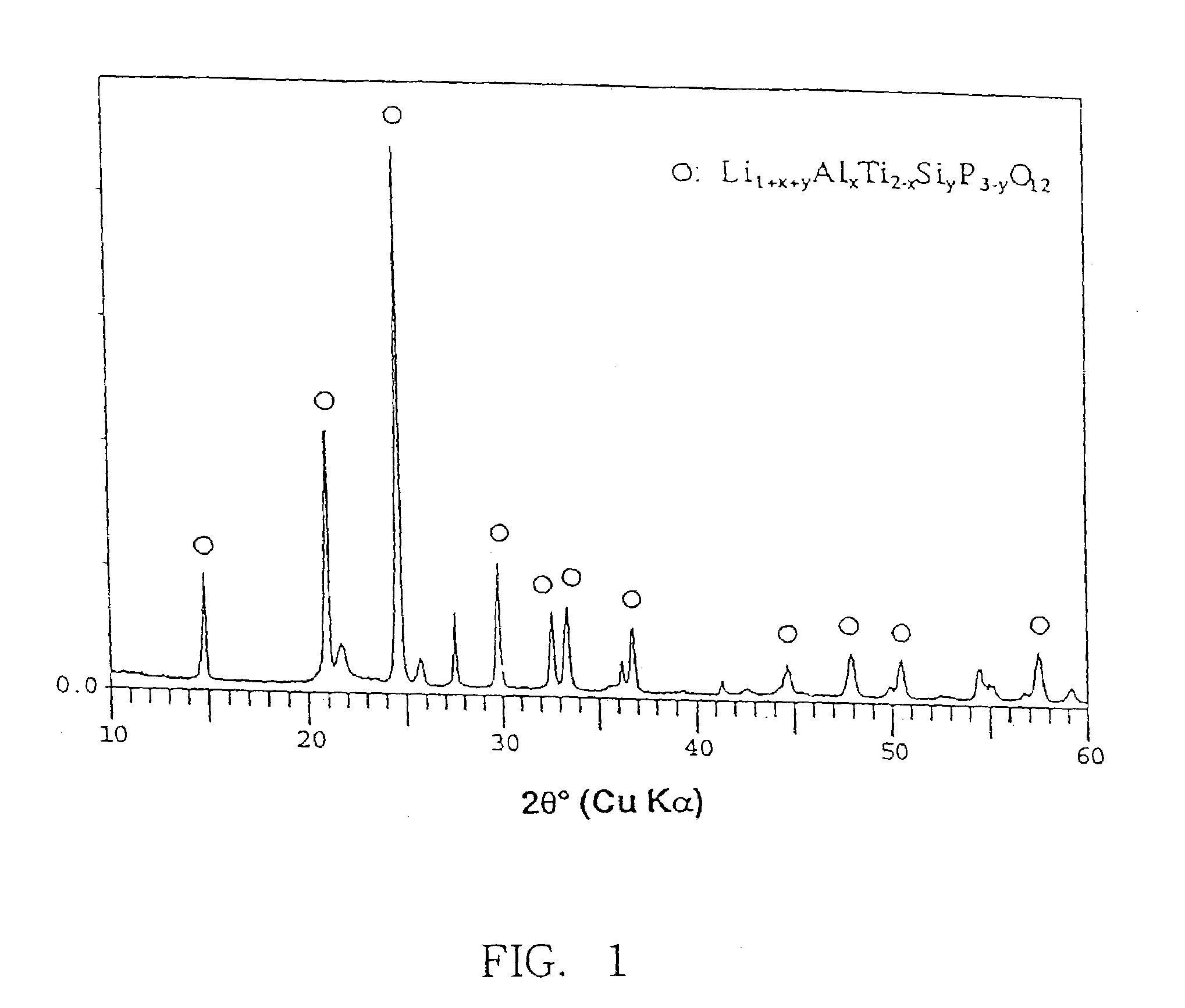
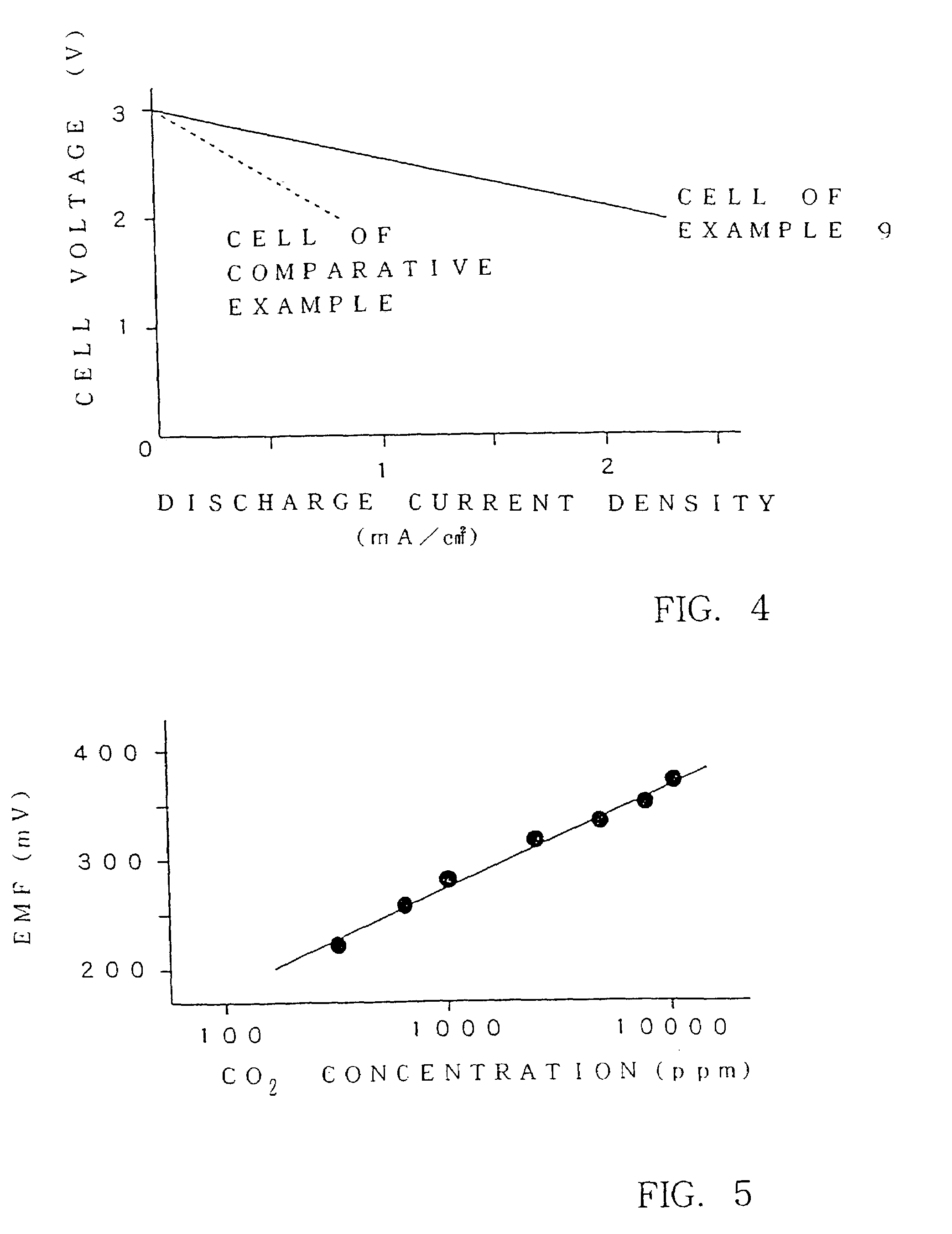
There are provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O538–40%TiO225–45%M2O3 (where M is Al or Ga) 5–15%Li2O10–20%and contain Li1+X(Al, Ga)XTi2−X(PO4)3 (where 0<X<0.8) as a main crystal phases. There are also provided glass-ceramics having a high lithium ion conductivity which include in mol %:P2O5 26–40%SiO20.5–12%TiO2 30–45%M2O3 (where M is Al or Ga) 5–10%Li2O 10–18%and contain Li1+X+YMXTi2−XSiYP3−YO12 (where 0<X≦0.4 and 0<Y≦0.6) as a main crystal phase. There are also provided solid electrolytes for an electric cell and a gas sensor using alkali ion conductive glass-ceramics, and a solid electric cell and a gas sensor using alkali ion conductive glass-ceramics as a solid electrolyte.
Owner:OHARA
Lithium-ion-conductive solid electrolyte and solid-electrolyte lithium battery
InactiveUS6277524B1Non-metal conductorsGallium/indium/thallium compoundsPhysical chemistryElectrolyte
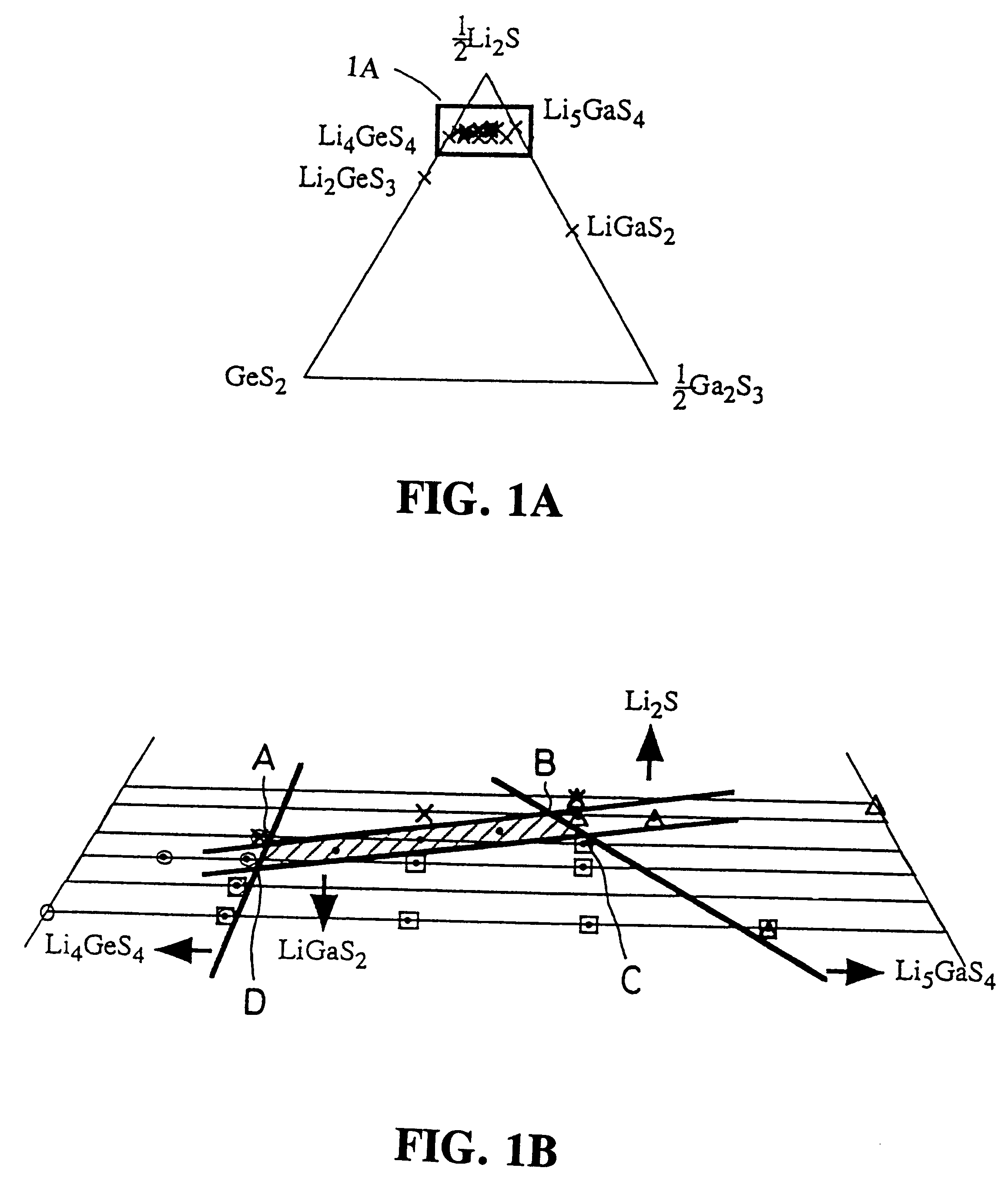
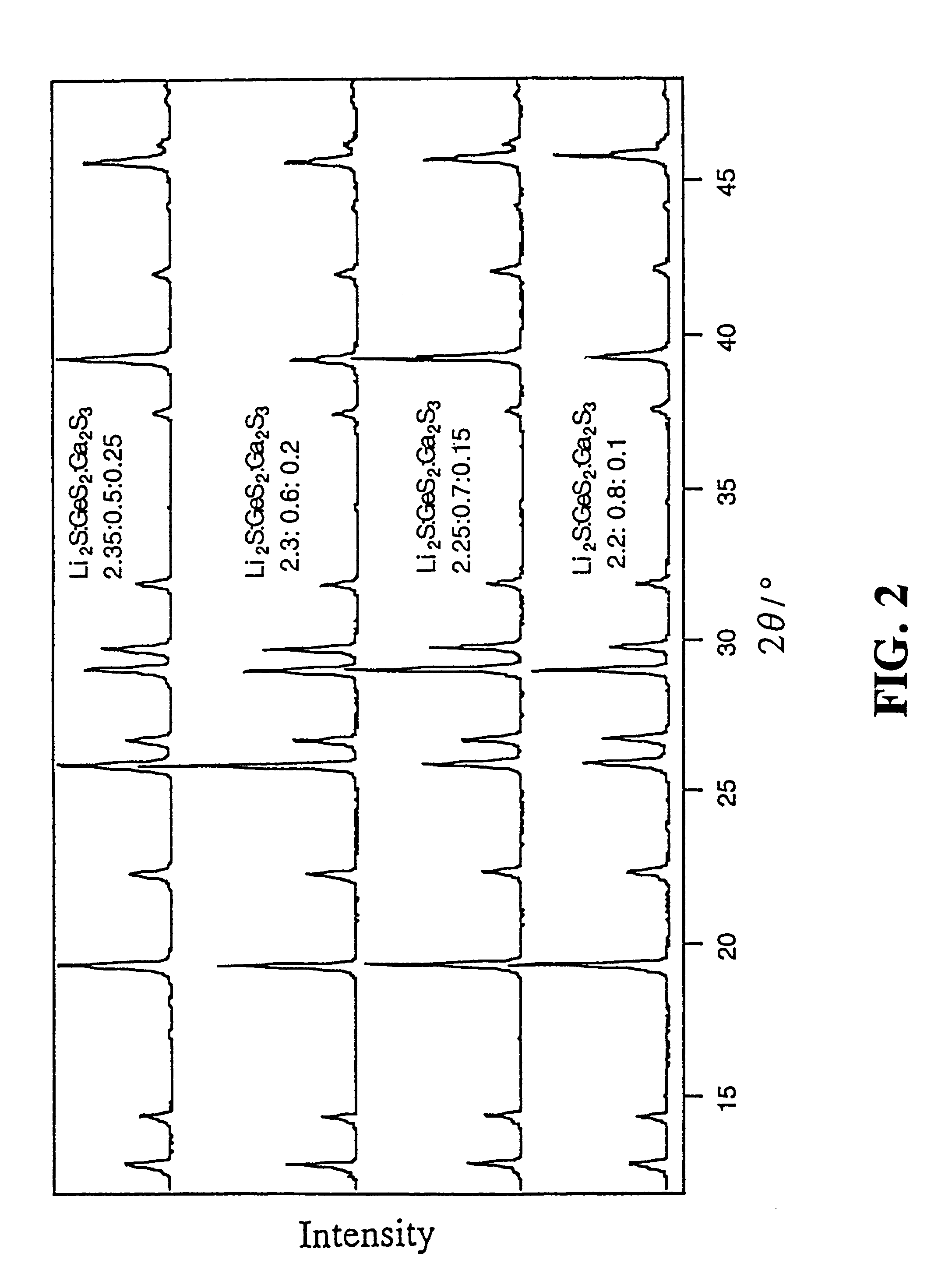
A lithium-ion-conductive solid electrolyte includes a lithium-ion-conductive substance expressed by a general formula Li2S-GeS2-X wherein "X" is at least one member selected from the group consisting of Ga2S3 and ZnS, or Li2S-SiS2-P2S5. It is superb in terms of stability and safety at elevated temperatures, since it is a crystalline solid of high ion conductivity. It can be applied to a solid electrolyte for lithium batteries.
Owner:TOYOTA JIDOSHA KK +1
Metal-air semi-fuel cell with an aqueous acid based cathode
InactiveUS20070259234A1Long time operationCell energy increasedFuel and primary cellsFuel and secondary cellsFuel cellsOxygen
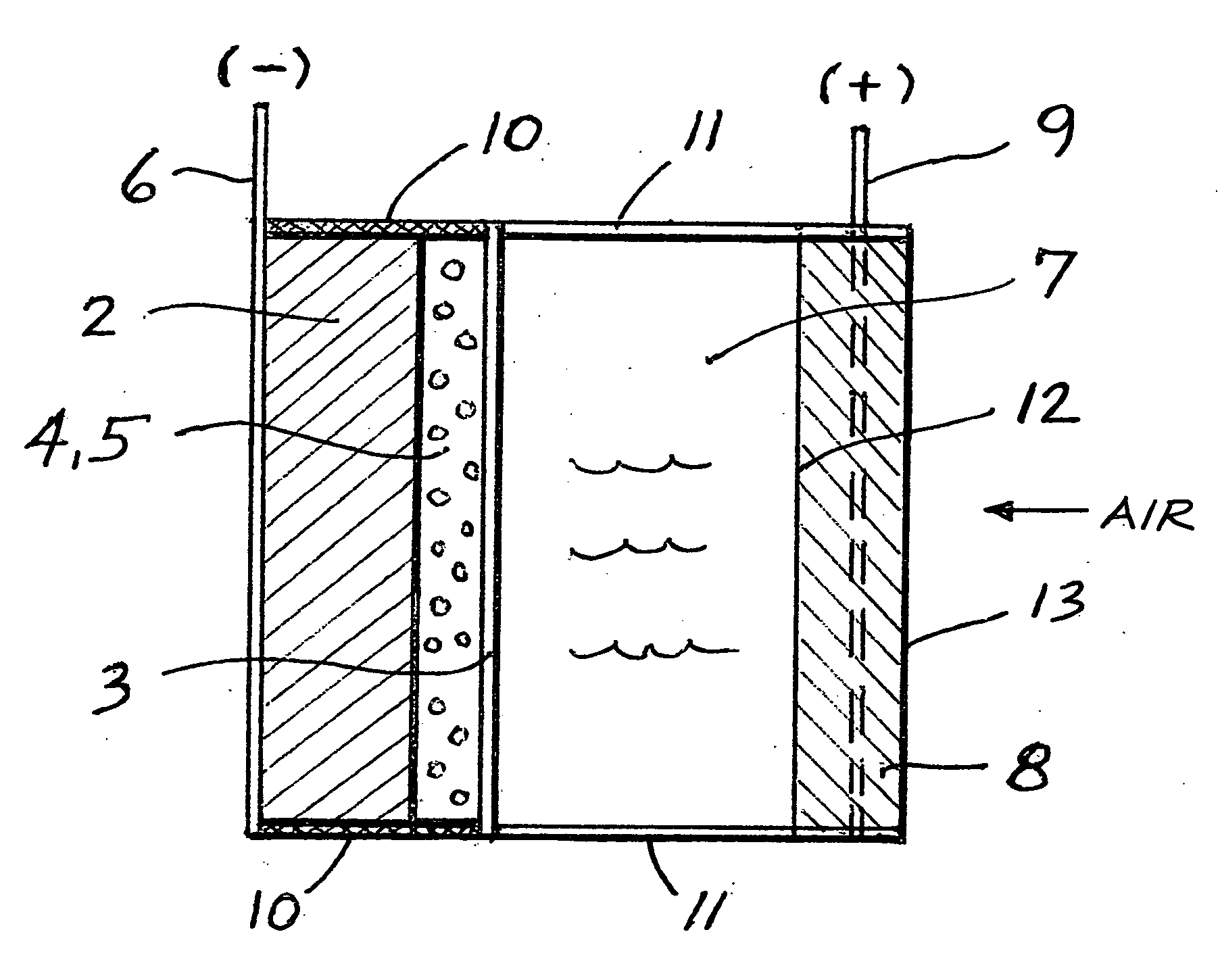
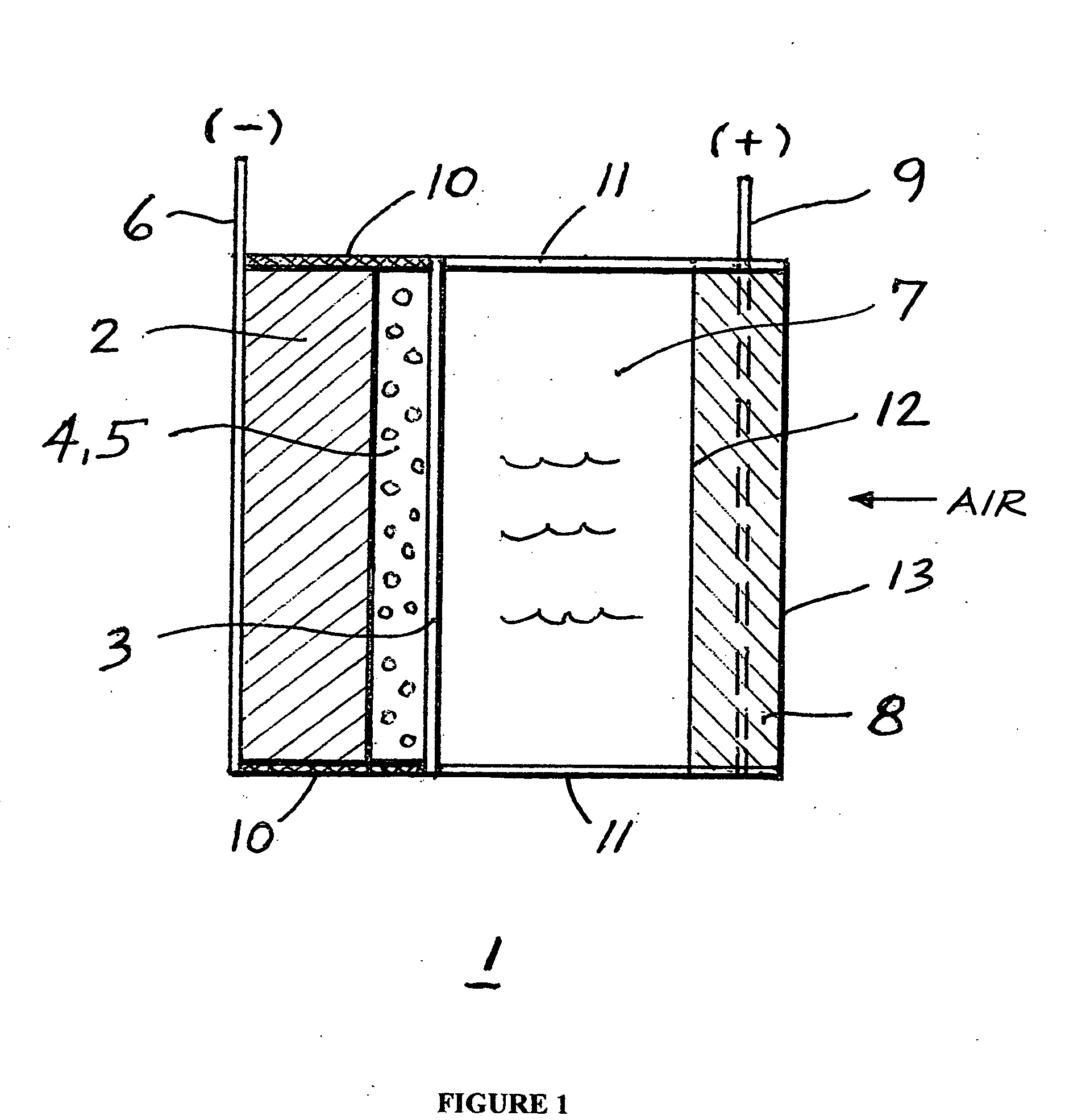
A metal-air semi-fuel cell is provided, preferably based on lithium anode and a fuel cell type air / oxygen electrode immersed in an aqueous neutral, alkali or acid solution. The lithium anode is comprised of the active metal and one or more separators protecting the anode from reacting with an aqueous solution. The outermost layer on the lithium electrode is a solid-state lithium-ion conducting glass-ceramic which is impervious to and stable towards aqueous solutions. The cathode is comprised of an air or oxygen fuel cell type electrode in contact with the aqueous solution. The lithium anode of this invention also can be replaced by other electroactive metals which react with water and acids, bases and neutral solutions, such as metals from Groups 1 and 2 of the Periodic Table of Elements in addition to Zn, Mg, and Al.
Owner:CHUA DAVID +2
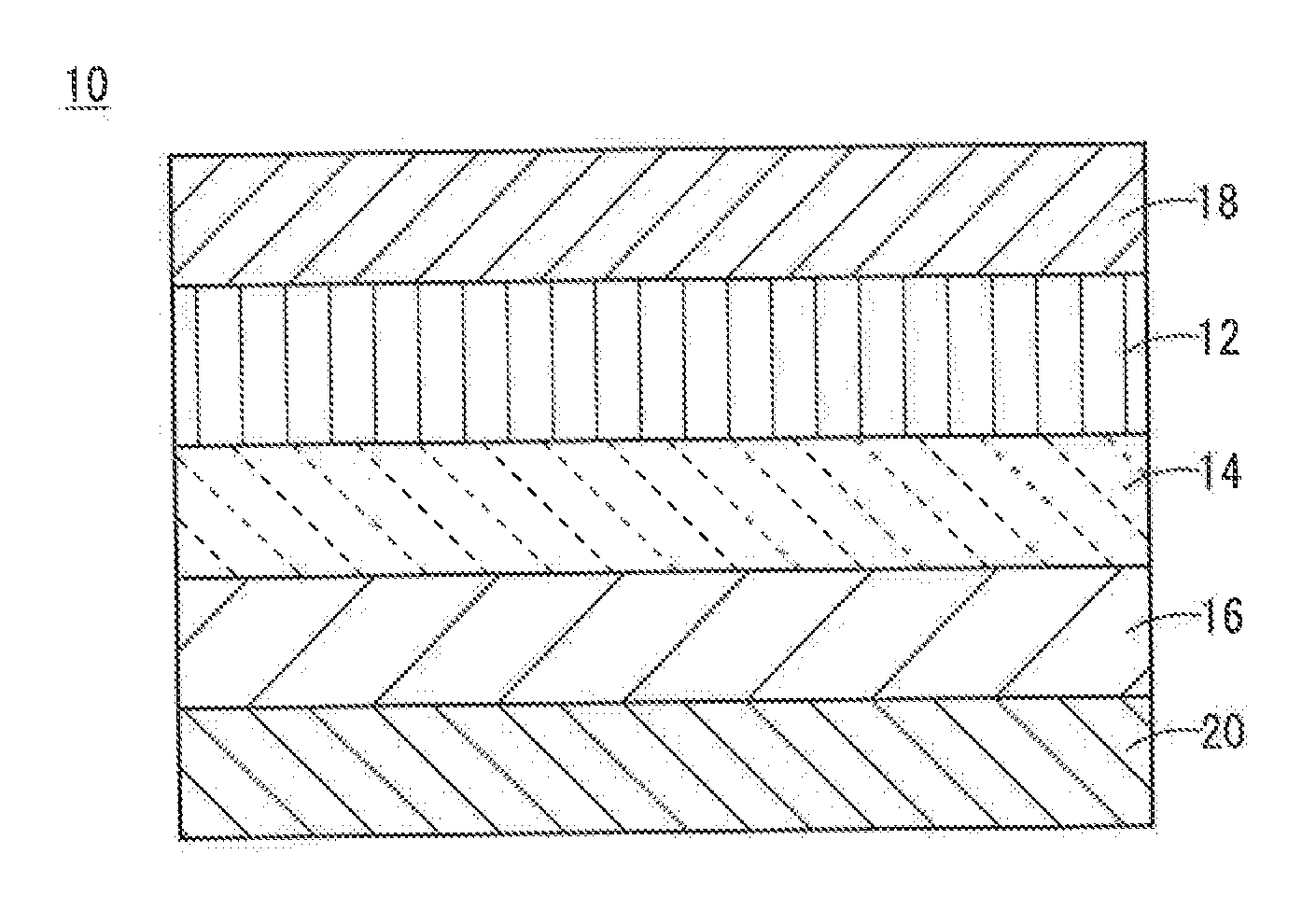
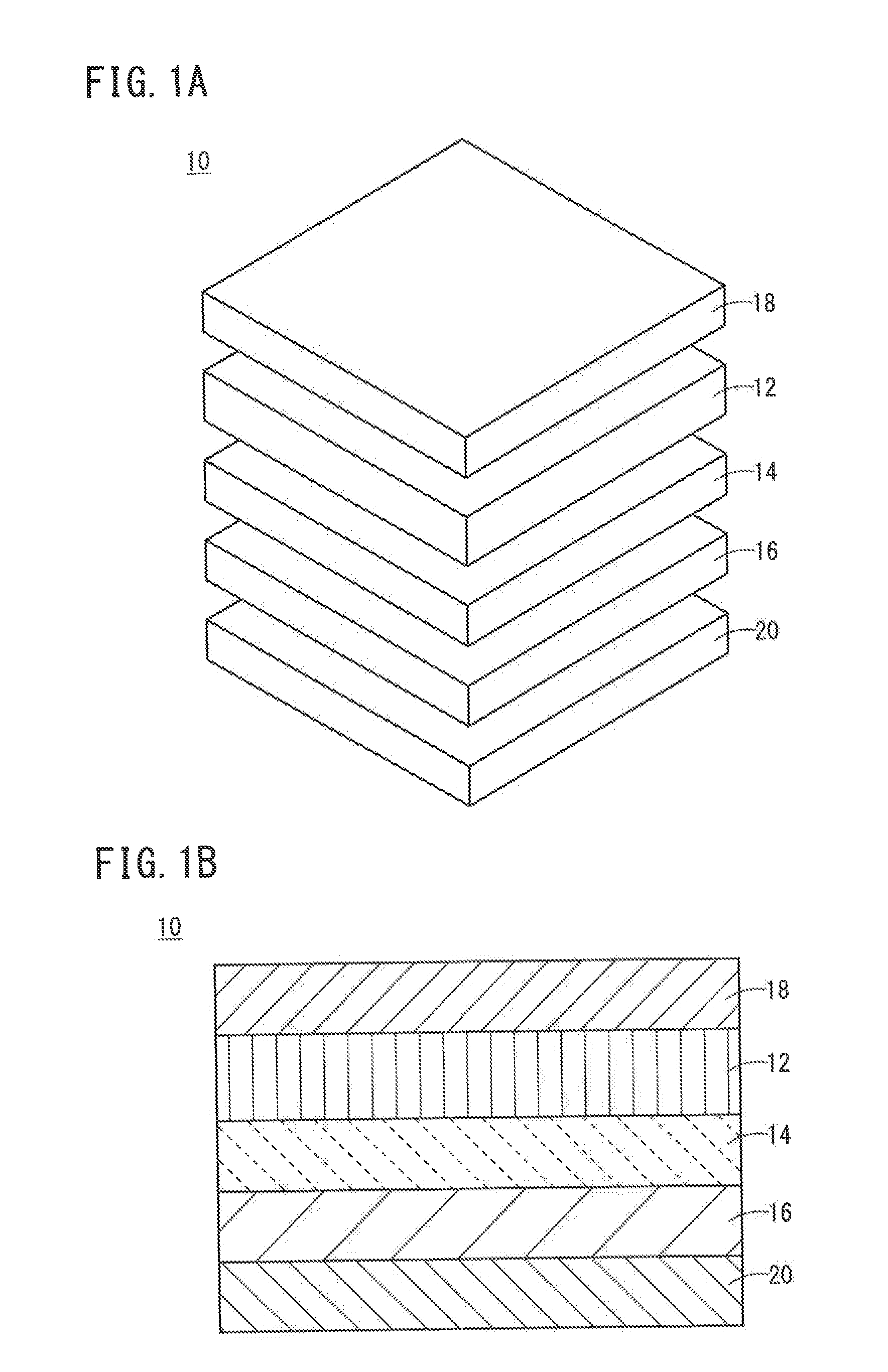
All-Solid-State Cell
InactiveUS20150037688A1Improve charge and discharge cycle characteristicsIncrease capacitySolid electrolytesElectrode thermal treatmentAll solid stateCrystal plane
An all-solid-state cell contains at least a positive electrode layer, a solid electrolyte layer, and a negative electrode layer, which are arranged in a stack. The positive electrode layer contains only a positive electrode active material, and a predetermined crystal plane of the positive electrode active material is oriented in a direction of lithium ion conduction. The negative electrode layer contains a carbonaceous material, and the volume ratio of the carbonaceous material to the negative electrode layer is 70% or greater.
Owner:NGK INSULATORS LTD
Lithium secondary battery
ActiveUS20060246355A1Increasing internal ion-conductivitySafely preventing short circuitFinal product manufactureSolid electrolyte cellsLithiumEngineering
A lithium secondary battery includes an electrode assembly having two electrodes and a separator interposed between the two electrodes, and a case for storing the electrode assembly, wherein the separator is formed by using a binder and a filler including a solid electrolyte having lithium ion conductivity. The lithium secondary battery has a separator and an electrolyte capable of increasing internal ion-conductivity. Also, a lithium secondary battery has a separator capable of safely preventing a short circuit between the electrodes in a possibly high temperature.
Owner:SAMSUNG SDI CO LTD
Lithium ion secondary battery and solid electrolyte therefor
InactiveUS20070048617A1Improve lithium ion conductivityEasy to handleNon-metal conductorsFinal product manufactureLithiumPhysical chemistry
A solid electrolyte and a lithium ion secondary battery A solid electrolyte for a lithium ion secondary battery has a laminate of at least two layers. The thickest layer of the laminate comprises lithium ion conductive crystalline, preferably lithium ion conductive glass-ceramics having a predominant layer of Li1+x+y(Al, Ga)x(Ti, Ge)2−xSiyP3−yO12 where 0≦x≦1, 0≦y≦1. In a preferred embodiment, thickness of an electrolyte layer comprising the lithium ion conductive glass-ceramics is 150 μm or below and thickness of an electrolyte layer which does not contain lithium ion conductive glass-ceramics or contains only a small amount of lithium ion conductive glass-ceramics is 50 μm or below.
Owner:OHARA
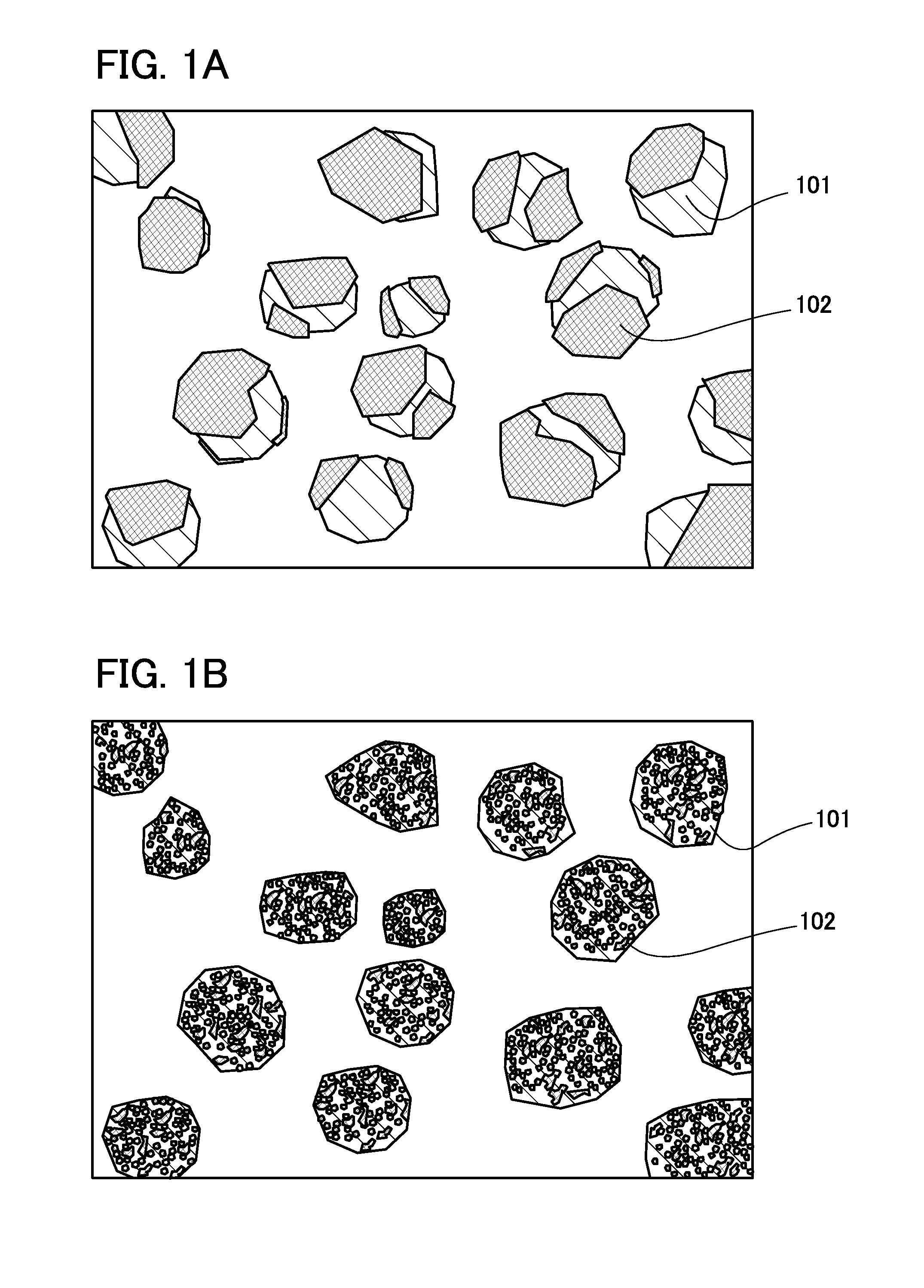
Negative electrode for power storage device, method for forming the same, and power storage device
ActiveUS20130266858A1Improve cycle performanceDecrease in capacity in chargeElectrode thermal treatmentGraphiteElectrical batteryEngineering
An object is to suppress electrochemical decomposition of an electrolyte solution and the like at a negative electrode in a lithium ion battery or a lithium ion capacitor; thus, irreversible capacity is reduced, cycle performance is improved, or operating temperature range is extended. A negative electrode for a power storage device including a negative electrode current collector, a negative electrode active material layer which is over the negative electrode current collector and includes a plurality of particles of a negative electrode active material, and a film covering part of the negative electrode active material. The film has an insulating property and lithium ion conductivity.
Owner:SEMICON ENERGY LAB CO LTD
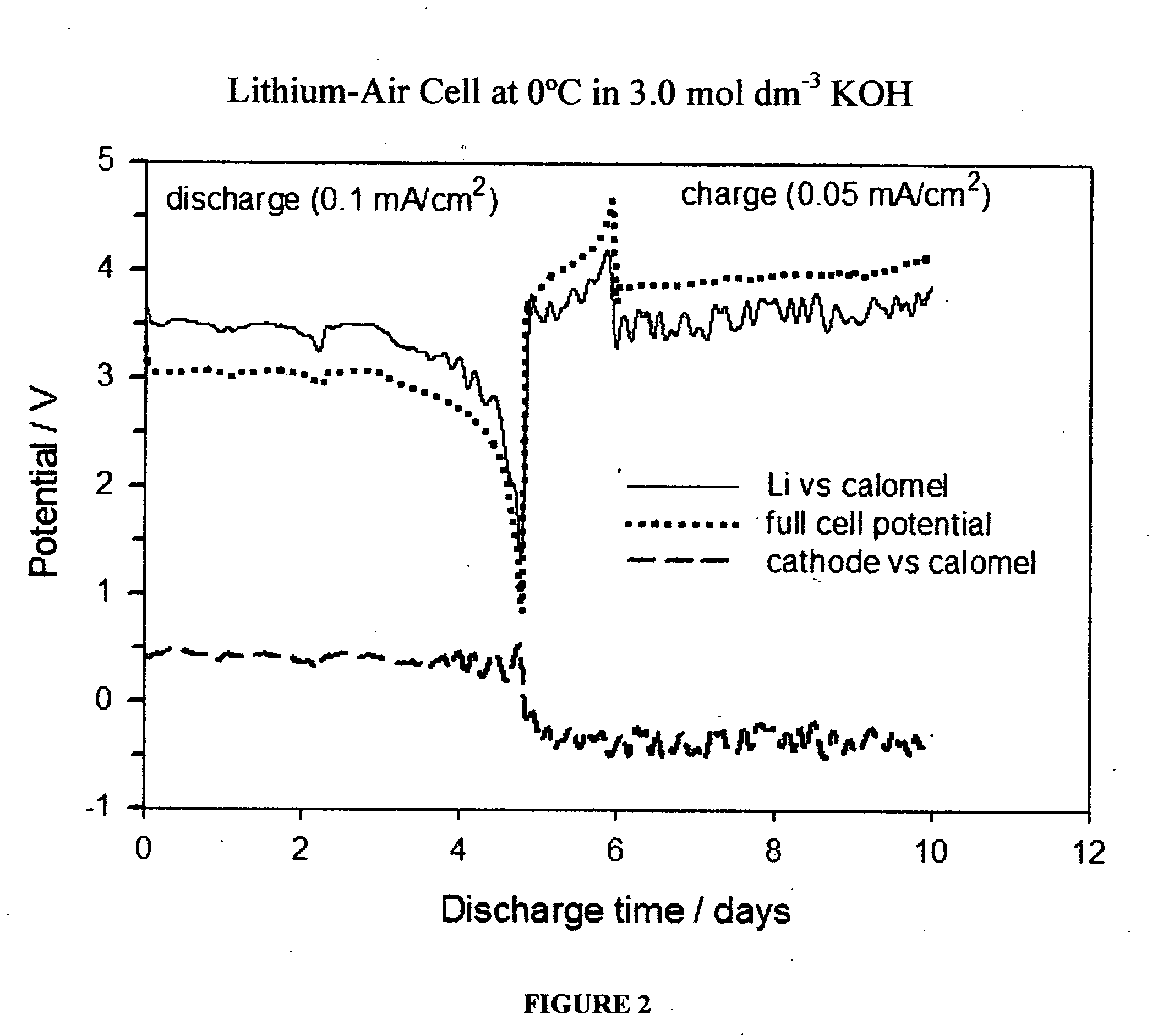
Lithium air secondary battery
ActiveUS20150024292A1Solve the blockageSolution to short lifeSolid electrolytesFuel and secondary cellsLithiumEngineering
The present invention provides a lithium-air secondary battery that is capable of effectively preventing deterioration of an alkaline electrolytic solution, air electrode, and negative electrode and has a long life and high long-term reliability. The lithium-air secondary battery comprises an air electrode 12 functioning as a positive electrode, an anion exchanger 14 provided in close contact with one side of the air electrode and composed of a hydroxide-ion conductive inorganic solid electrolyte, a separator 16 provided away from the anion exchanger and composed of a lithium-ion conductive inorganic solid electrolyte, a negative electrode 18 provided so as to be capable of supplying and receiving lithium ions to and from the separator and comprising lithium, and an alkaline electrolytic solution 20 filled between the anion exchanger and the separator.
Owner:NGK INSULATORS LTD
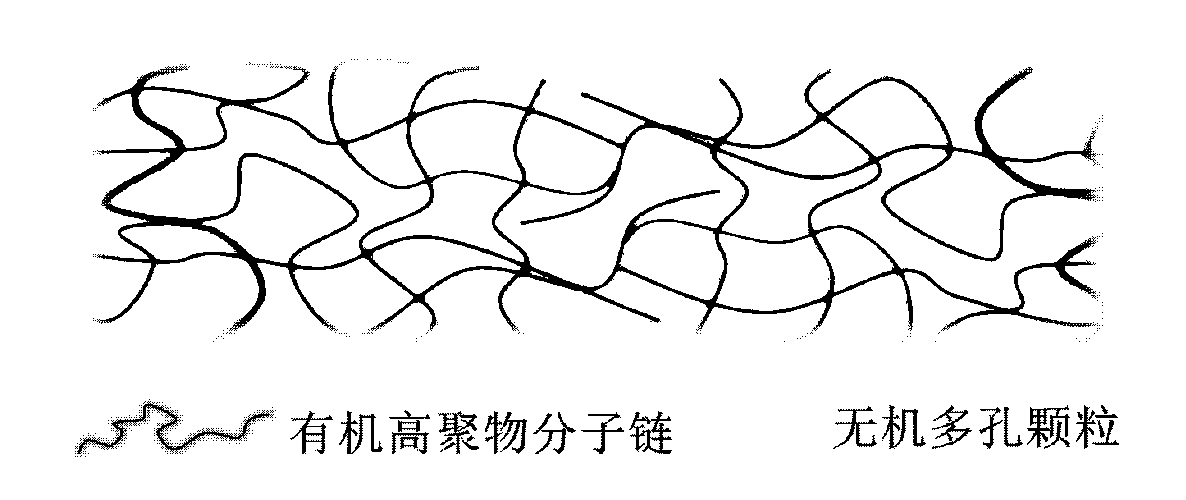
Organic-inorganic composite membrane as well as preparation and application thereof
ActiveCN103311486AChange structureImprove thermal stabilityCell component detailsLithium-ion batteryMechanical property
The invention discloses an organic-inorganic composite membrane as well as preparation and application thereof, and belongs to the technical field of preparation of a lithium ion battery material. The organic-inorganic composite membrane is prepared from inorganic particles and a high-molecular polymer, wherein the inorganic particles are evenly embedded and distributed inside the high-molecular polymer, and are selected from layered inorganic materials and / or porous inorganic materials; the mass ratio of the inorganic particles to the high-molecular polymer is (5-20):1; the particle sizes of the inorganic particles are 2-100nm. The organic-inorganic composite membrane disclosed by the invention has good mechanical property and lithium ionic conduction ability after absorbing electrolyte; the composite membrane has excellent safety performance after being prepared into an electric element, and is suitable for being applied to the field of a large battery, especially a large fixation energy storage battery. The organic-inorganic composite membrane is simple and feasible in preparation technology, and conveniently achieves industrial production.
Owner:王海斌
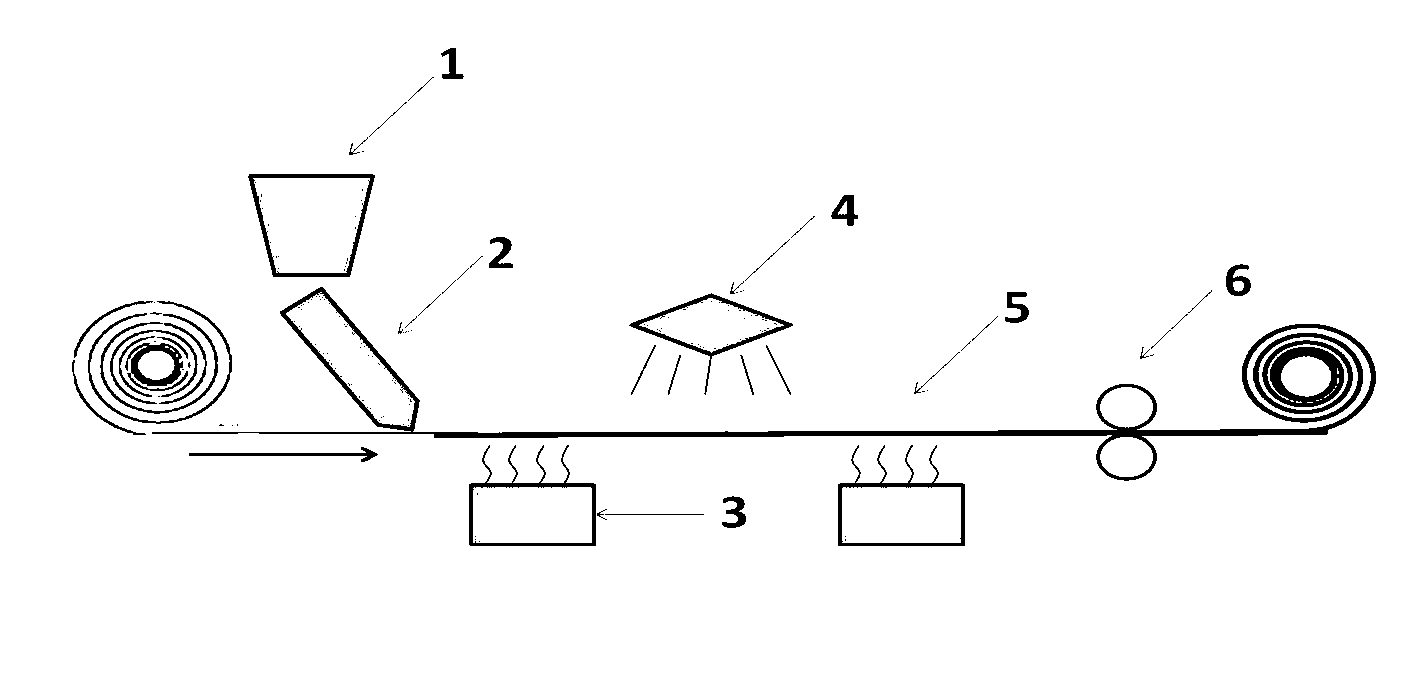
Composite quasi-solid-state electrolyte and preparation method thereof, and lithium battery or lithium ion battery containing composite quasi-solid-state electrolyte
InactiveCN107645013AImprove mechanical propertiesAddress volume expansionFinal product manufactureLi-accumulatorsSolid state electrolyteInterfacial resistance
The present invention provides a composite quasi-solid-state electrolyte, a composite quasi-solid-state electrolyte membrane, preparation methods of composite quasi-solid-state electrolyte and the composite quasi-solid-state electrolyte membrane, and a lithium battery or a lithium ion battery containing the composite quasi-solid-state electrolyte membrane. The composite quasi-solid-state electrolyte comprises a solid electrolyte, a lithium salt-containing liquid electrolyte, inorganic nanoparticles and a binder, wherein the static electricity or functional groups on the surface of the inorganic nanoparticles can adsorb the electrolyte so as to make the composite quasi-solid-state electrolyte have strong adsorption capacity and strong liquid retention ability, and the inorganic nanoparticles can adsorb the lithium salt so as to change the lithium ion conduction mechanism, reduce the interfacial resistance between the liquid electrolyte and the solid-state electrolyte, change the deposition morphology of lithium, hinder the formation of lithium dendrite, and reduce the pulverization of lithium. In addition, by adding the solid electrolyte, the composite quasi-solid-state electrolyteof the present invention can maintain the high conductivity and can effectively reduce the content of the liquid electrolyte so as to improve the safety of the battery.
Owner:BEIJING WELION NEW ENERGY TECH CO LTD
Lithium battery
InactiveUS20090068563A1Suppression problemOvercome lack of heat resistanceFinal product manufactureElectrode carriers/collectorsSulfideVapor phase
A lithium battery includes a substrate, a positive electrode layer, a negative electrode layer, and a sulfide solid electrolyte layer disposed between the positive electrode layer and the negative electrode layer, the positive electrode layer, the negative electrode layer, and the sulfide solid electrolyte layer being provided on the substrate. In this lithium battery, the positive electrode layer is formed by a vapor-phase deposition method, and a buffer layer that suppresses nonuniformity of distribution of lithium ions near the interface between the positive electrode layer and the sulfide solid electrolyte layer is provided between the positive electrode layer and the sulfide solid electrolyte layer. As the buffer layer, a lithium-ion conductive oxide, in particular, LixLa(2-x) / 3TiO3 (x=0.1 to 0.5), Li7+xLa3Zr2O12+(x / 2) (−5≦×≦3, preferably −2≦×≦2), or LiNbO3 is preferably used.
Owner:SUMITOMO ELECTRIC IND LTD
All solid lithium ion secondary battery and a solid electrolyte therefor
ActiveUS20070048619A1Sufficient effectSmall particle sizeSolid electrolyte cellsElectrolytesLithiumElectrical battery
An all solid type lithium ion secondary battery which has high heat resistance and can be used over a broad temperature range, has a high battery capacity and an excellent charging discharging characteristic, and can be used stably for a long period of time includes an inorganic substance including a lithium ion conductive crystalline and is substantially free of an organic substance and an electrolytic solution. The inorganic substance comprising a lithium ion conductive crystalline preferably is lithium ion conductive glass-ceramics.
Owner:OHARA
Nonaqueous electrolyte secondary battery and method for producing same
InactiveUS20060147800A1Conductivity is deterioratedImprove charge and discharge cycle characteristicsPrimary cellsElectrode carriers/collectorsLithiumEngineering
A nonaqueous electrolyte secondary battery with excellent charging / discharging cycle characteristics is provided, more specifically a nonaqueous electrolyte secondary battery in which deterioration of the conductivity of a negative electrode due to charging / discharging cycle is suppressed and a method for manufacturing the same are provided. The nonaqueous electrolyte secondary battery includes: a positive electrode and a negative electrode that are capable of reversibly absorbing and desorbing Li ions; and a nonaqueous electrolyte having lithium ion conductivity. The negative electrode includes a collector and active material particles that are disposed on a surface of the collector. The active material particles include Si and at least one element R selected from the group consisting of Sn, In, Ga, Pb and Bi. Metallic bond including the element R is formed between the active material particles.
Owner:PANASONIC CORP
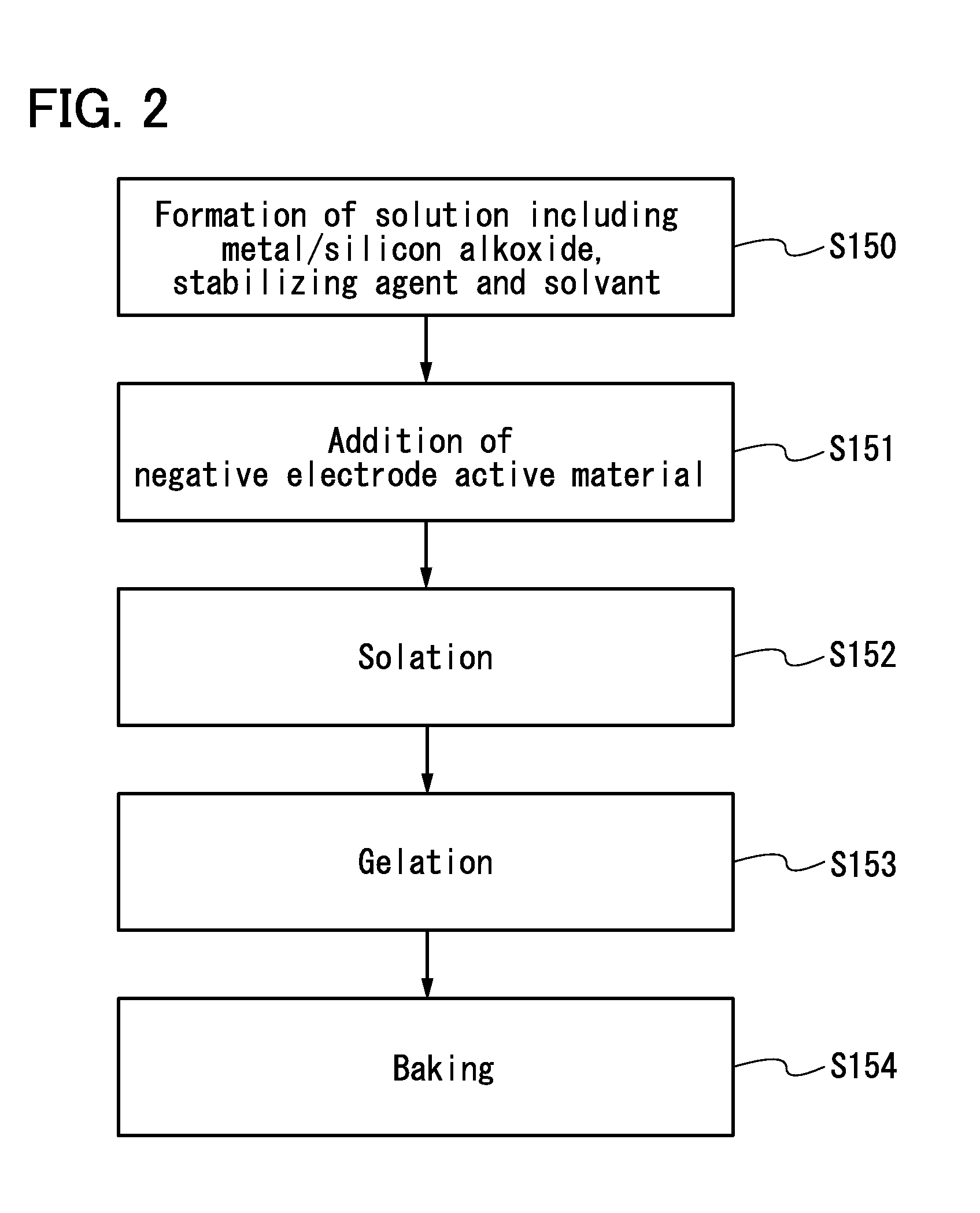
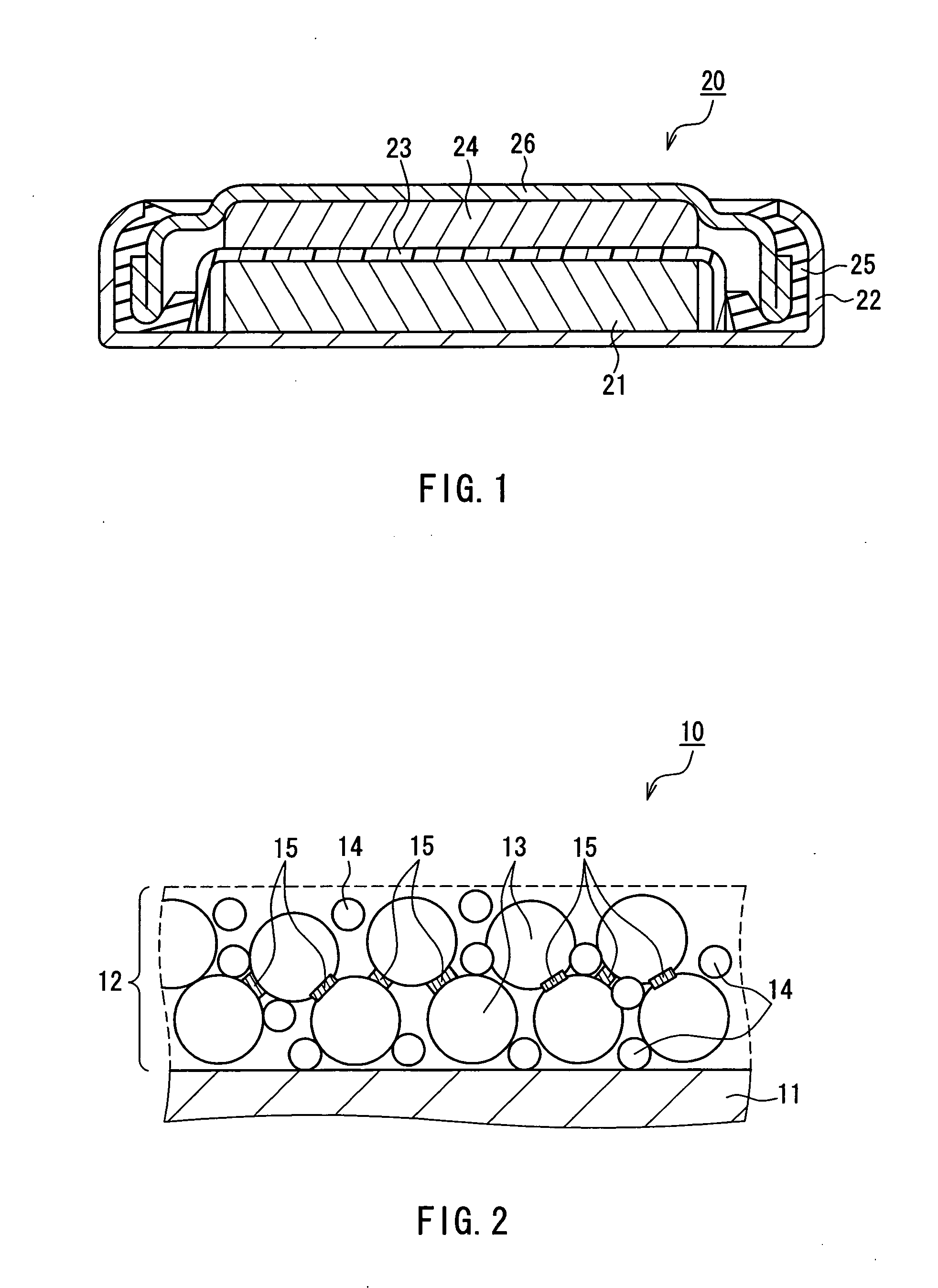
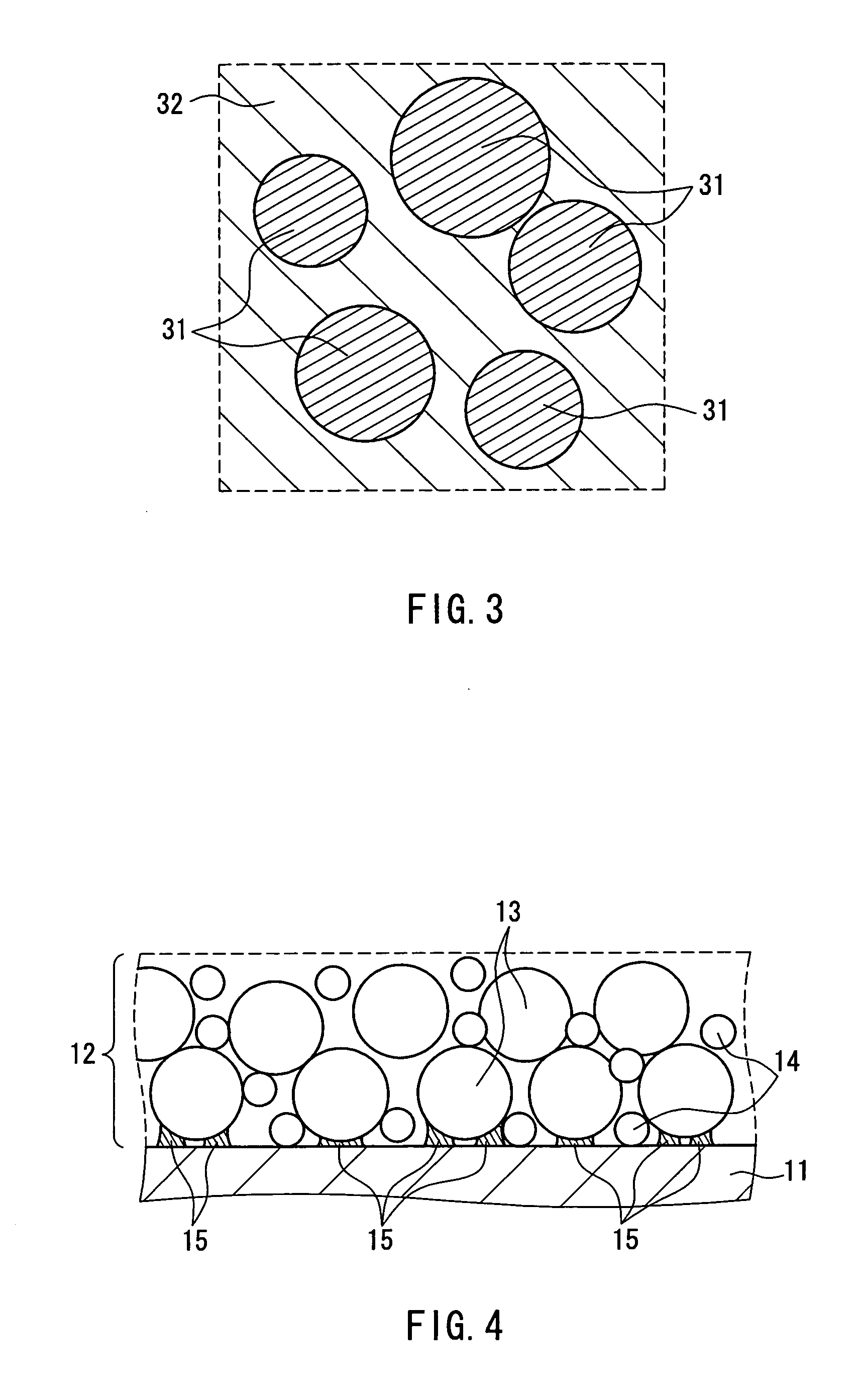
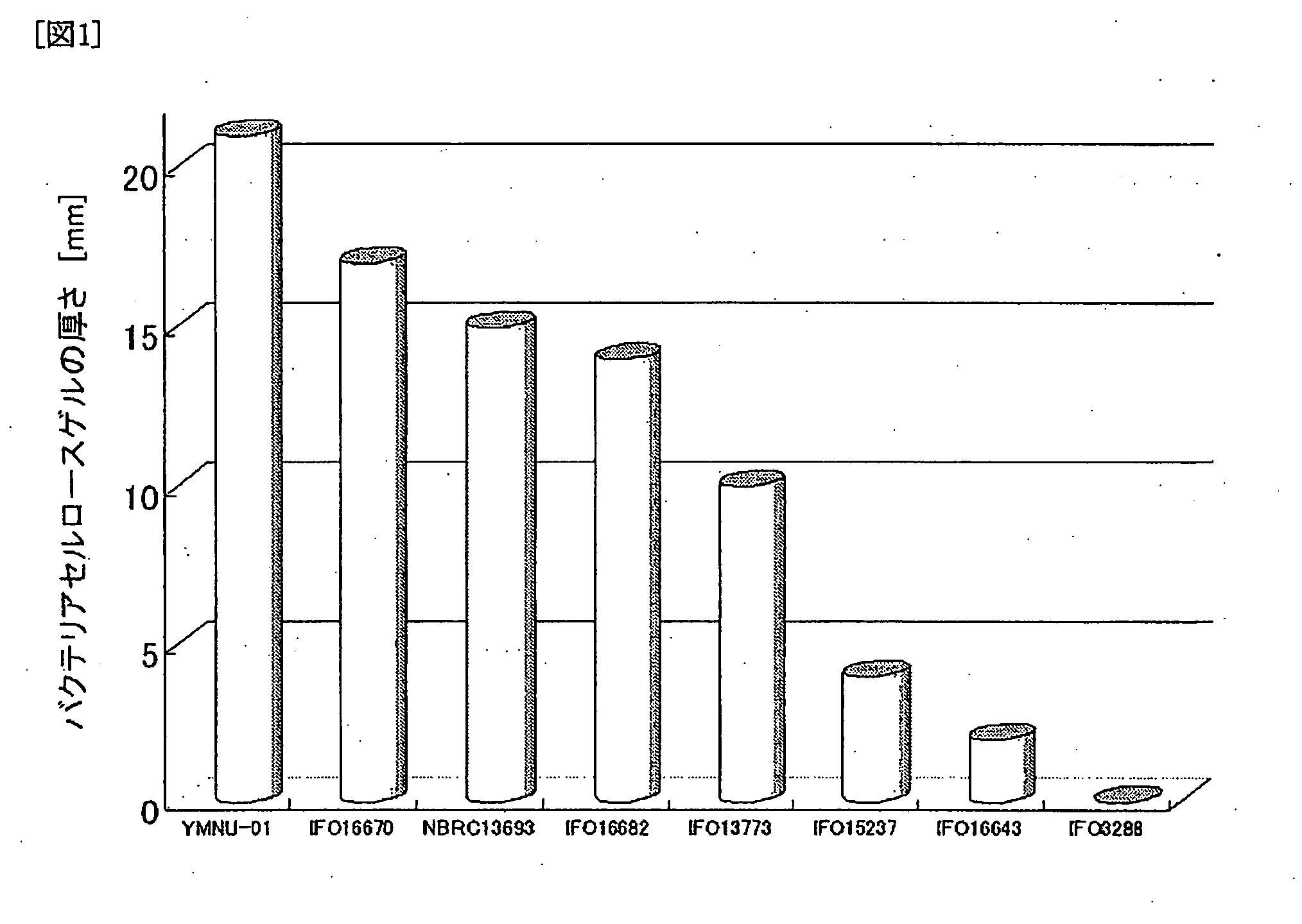
Lithium Ion Conductive Material Utilizing Bacterial Cellulose Organogel, Lithium Ion Battery Utilizing the Same and Bacterial Cellulose Aerogel
InactiveUS20080220333A1High mechanical strengthImprove performanceMaterial nanotechnologyConductive materialConductive materialsSolvent
A lithium ion conductive material that excels in mechanical strength, exhibiting high ion conductivity; a bacterial cellulose composite material having an inorganic material and / or organic material incorporated therein; and a bacterial cellulose aerogel. The water of bacterial cellulose hydrogel is replaced by a nonaqueous solvent containing a lithium compound. Bacterial cellulose producing bacteria are grown in a culture medium having an inorganic material and / or organic material added thereto. The bacterial cellulose hydrogel is dehydrated and dried.
Owner:YANO SHOICHIRO +5
Solid-state battery and method for manufacturing electrode active material
ActiveUS20160204466A1Improve ionic conductivityImprove stabilitySolid electrolytesElectrode thermal treatmentSulfurSolid-state battery
One embodiment provides a solid-state battery that has a positive-electrode layer; a negative-electrode layer; and a lithium-ion-conducting solid electrolyte layer disposed between the positive-electrode layer and the negative-electrode layer. The positive-electrode layer contains a positive-electrode active material and a solid electrolyte comprising a hydride of a complex. Said positive-electrode active material is sulfur-based, and the solid electrolyte layer contains a solid electrolyte comprising a hydride of a complex.
Owner:MITSUBISHI GAS CHEM CO INC +1
Non-aqueous electrolyte, rechargeable lithium battery, and rechargeable battery system
ActiveUS20060078801A1High ion conductivityGood voltage resistanceCell electrodesOrganic electrolyte cellsLithium metalArame
A non-aqueous electrolyte having improved lithium ion conductivity and excellent voltage resistance is provided. A lithium rechargeable battery and a rechargeable battery system including the inventive non-aqueous electrolyte is also provided. The non-aqueous electrolyte includes at least one aromatic compound which is polymerizable at a working electrode potential of 4.2 to 4.5 V when a lithium metal is used as a counter electrode and platinum is used as a working electrode.
Owner:SAMSUNG SDI CO LTD
All-solid-state cell
InactiveUS20140072870A1Accelerate emissionsIncrease battery capacitySolid electrolytesActive material electrodesAll solid stateCarbon nanotube
An all-solid-state cell has a positive electrode layer containing a positive electrode active material, a solid electrolyte layer containing a lithium ion conducting material, and a negative electrode layer containing a negative electrode active material. The negative electrode active material in the negative electrode layer contains a plurality of cylindrical carbon nanotube molecules, and the axes of the carbon nanotube molecules are oriented in a predetermined direction.
Owner:NGK INSULATORS LTD
Sulfide-based lithium-ion-conducting solid electrolyte glass, all-solid lithium secondary battery, and method for manufacturing all-solid lithium secondary battery
ActiveUS20090142669A1Promote crystallizationReduce processing timePot furnacesFinal product manufactureLithiumOptoelectronics
Owner:SEIKO EPSON CORP
Standalone sulfide based lithium ion-conducting glass solid electrolyte and associated structures, cells and methods
ActiveUS20160156065A1Improve performanceEasy to adaptSolid electrolytesGlass forming apparatusPhysical chemistryLithium-ion battery
A standalone lithium ion-conductive solid electrolyte including a freestanding inorganic vitreous sheet of sulfide-based lithium ion conducting glass is capable of high performance in a lithium metal battery by providing a high degree of lithium ion conductivity while being highly resistant to the initiation and / or propagation of lithium dendrites. Such an electrolyte is also itself manufacturable, and readily adaptable for battery cell and cell component manufacture, in a cost-effective, scalable manner.
Owner:POLYPLUS BATTERY CO INC
Positive electrode active materials for secondary batteries and methods of preparing same
InactiveUS6878490B2Deliver in short periodRetake in short periodAluminium compoundsAlkali titanatesPower capabilityLithium metal
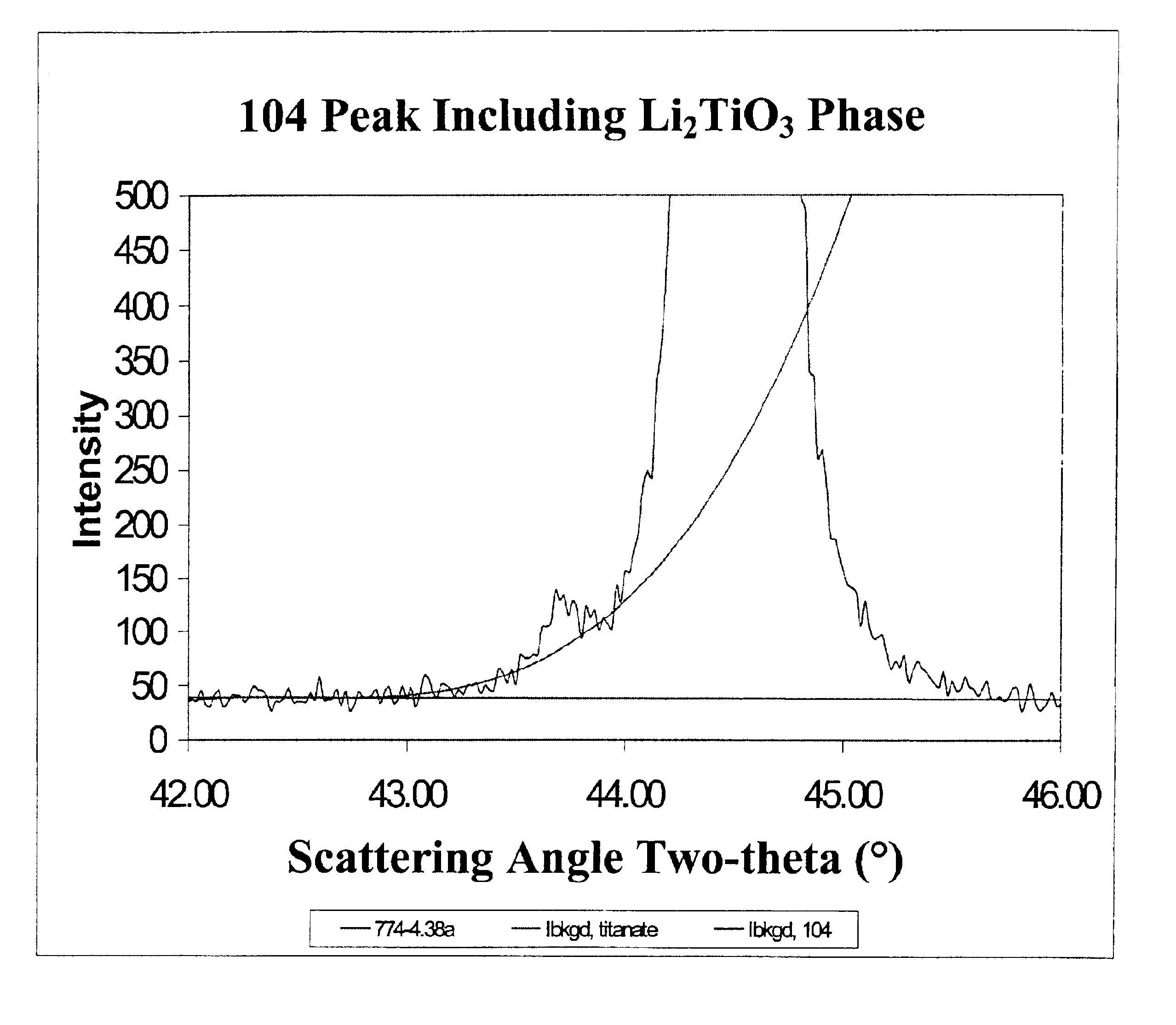
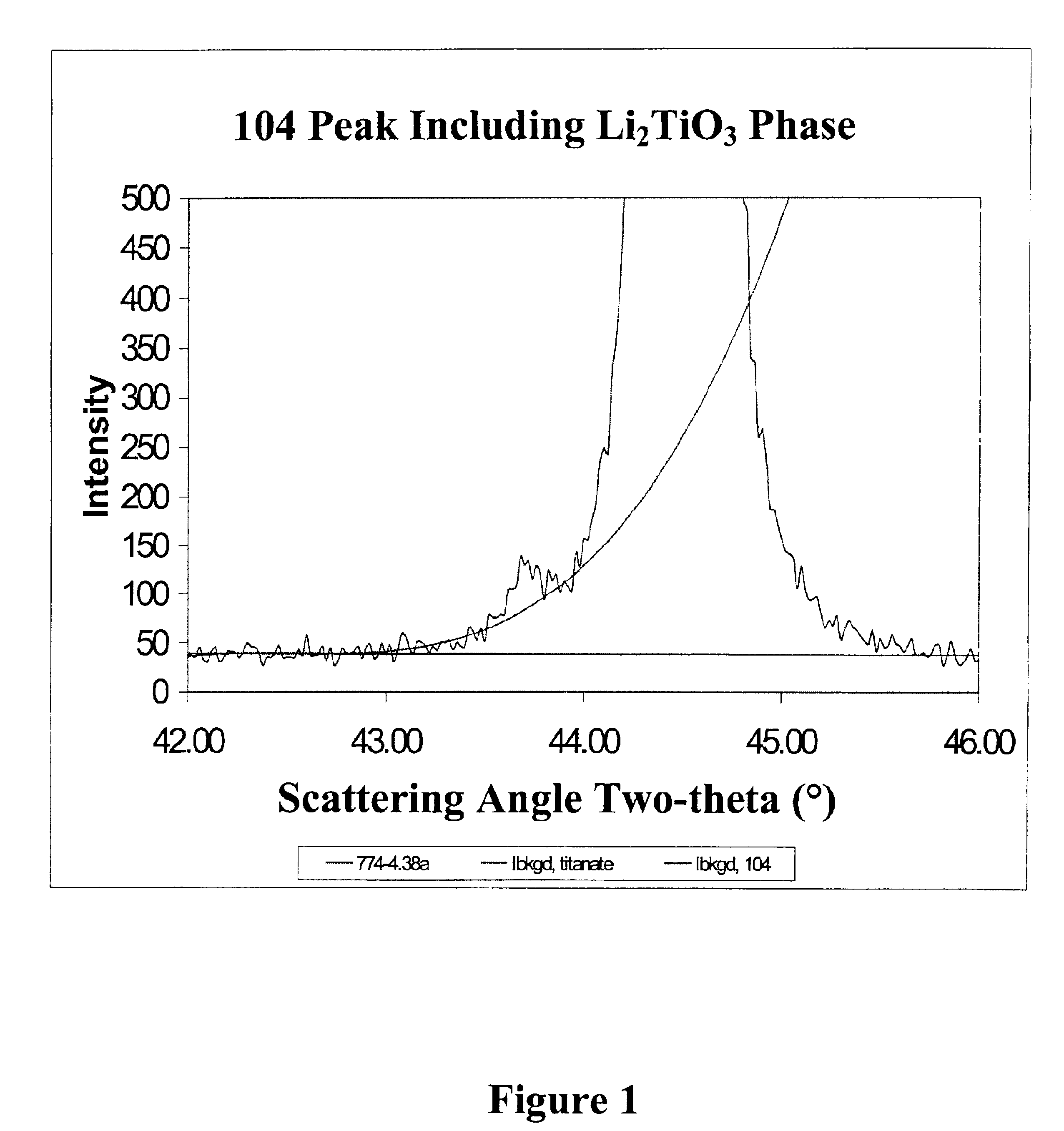
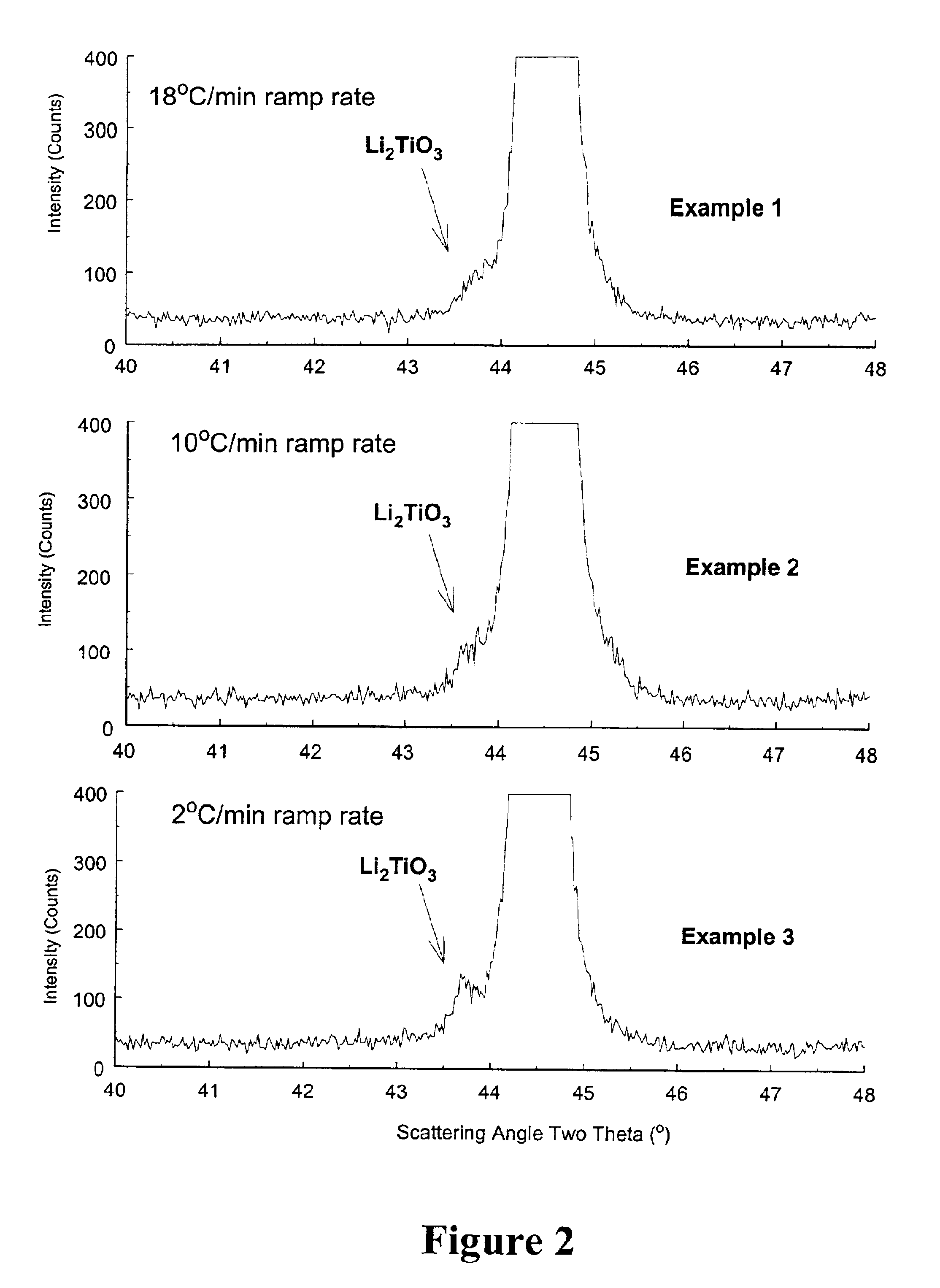
The present invention is a positive electrode active material that can be used in secondary lithium and lithium-ion batteries to provide the power capability, i.e., the ability to deliver or retake energy in short periods of time, desired for large power applications such as power tools, electric bikes and hybrid electric vehicles. The positive electrode active material of the invention includes at least one electron conducting compound of the formula LiM1x−y{A}yOz and at least one electron insulating and lithium ion conducting lithium metal oxide, wherein M1 is a transition metal, {A} is represented by the formula ΣwiBi wherein Bi is an element other than M1 used to replace the transition metal M1 and wi is the fractional amount of element Bi in the total dopant combination such that Σwi=1; Bi is a cation in LiM1x−y{A}yOz; 0.95≦x≦2.10; 0≦y≦x / 2; and 1.90≦z≦4.20. Preferably, the lithium metal oxide is LiAlO2 or Li2M2O3 wherein M2 is at least one tetravalent metal selected from the group consisting of Ti, Zr, Sn, Mn, Mo, Si, Ge, Hf, Ru and Te. The present invention also includes methods of making this positive electrode active material.
Owner:UMICORE AG & CO KG
Laminate including active material layer and solid electrolyte layer, and all solid lithium secondary battery using the same
InactiveCN101076914AImprove life characteristicsIncrease capacityElectrode manufacturing processesSmall-sized cells cases/jacketsLithiumX-ray
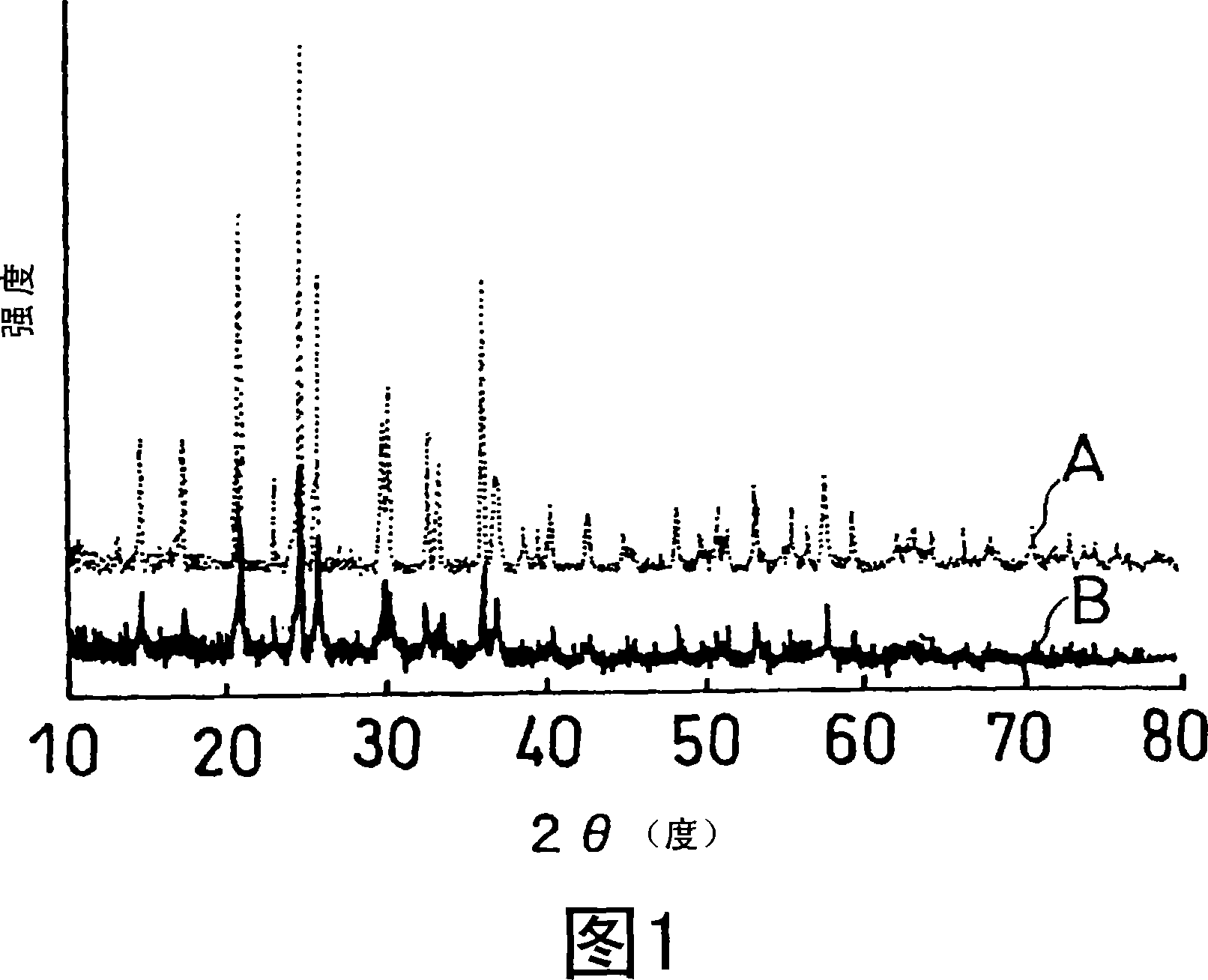
Disclosed is a multilayer body comprising an active material layer and a solid electrolyte layer which is bonded to the active material layer by sintering. The active material layer contains a crystalline first substance capable of discharging and adsorbing lithium ions, and the solid electrolyte layer contains a crystalline second substance having lithium ion conductivity. Incidentally, when this multilayer body is analyzed by an X-ray diffraction method, there is detected no component other than the constituents of the active material layer and the constituents of the solid electrolyte layer. Also disclosed is an all-solid lithium secondary battery comprising such a multilayer body and a negative electrode active material layer.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com