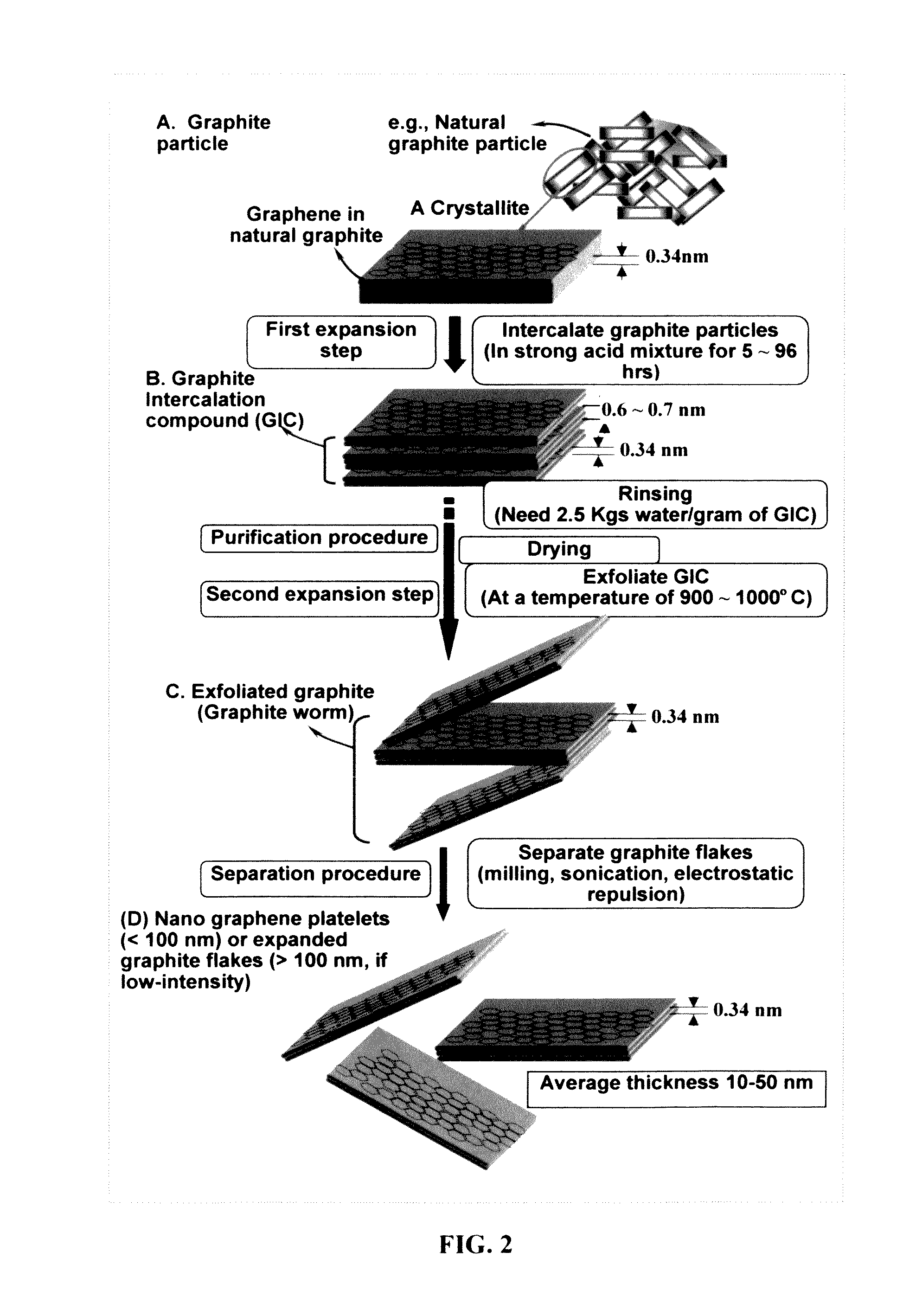
Patents
Literature
1448results about "Vapour deposition manufacturing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
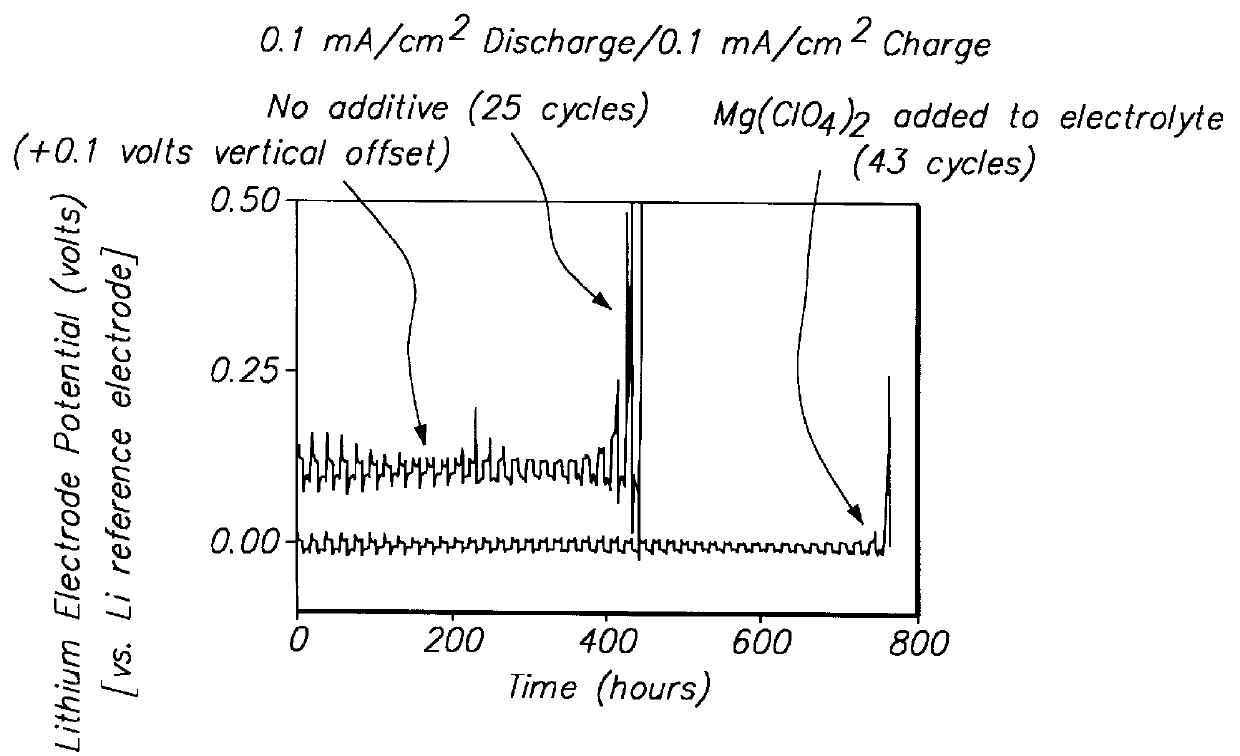
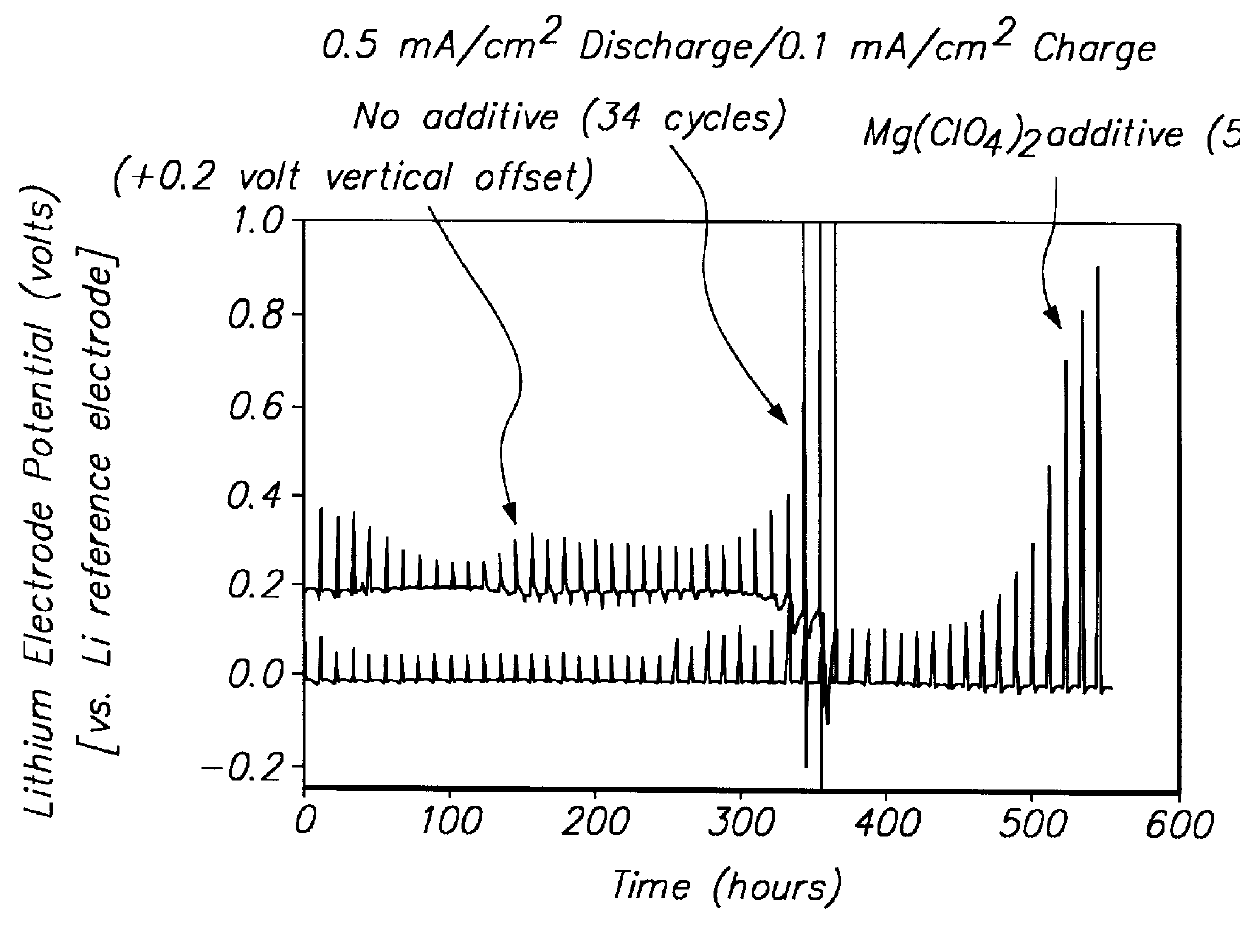
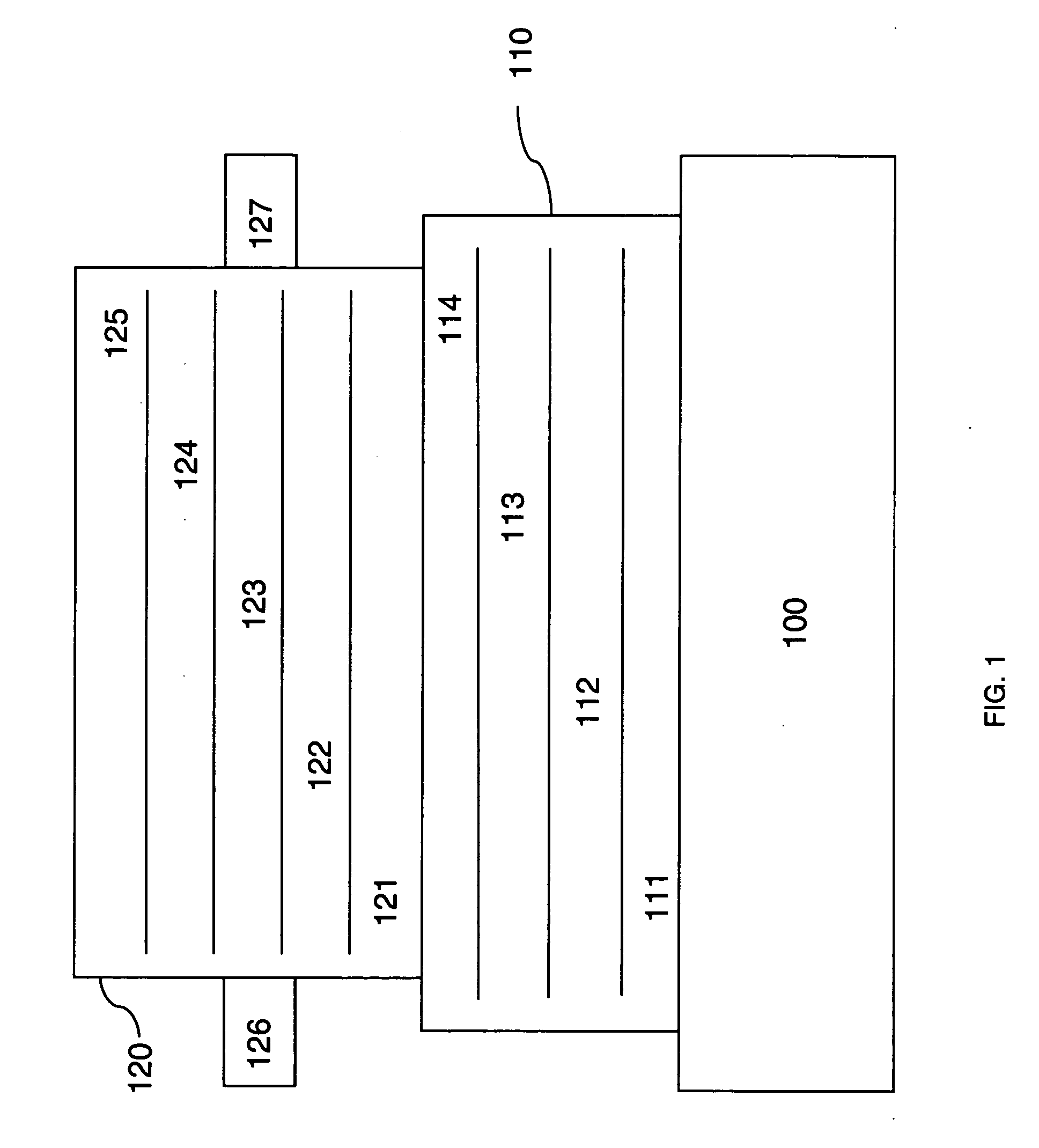
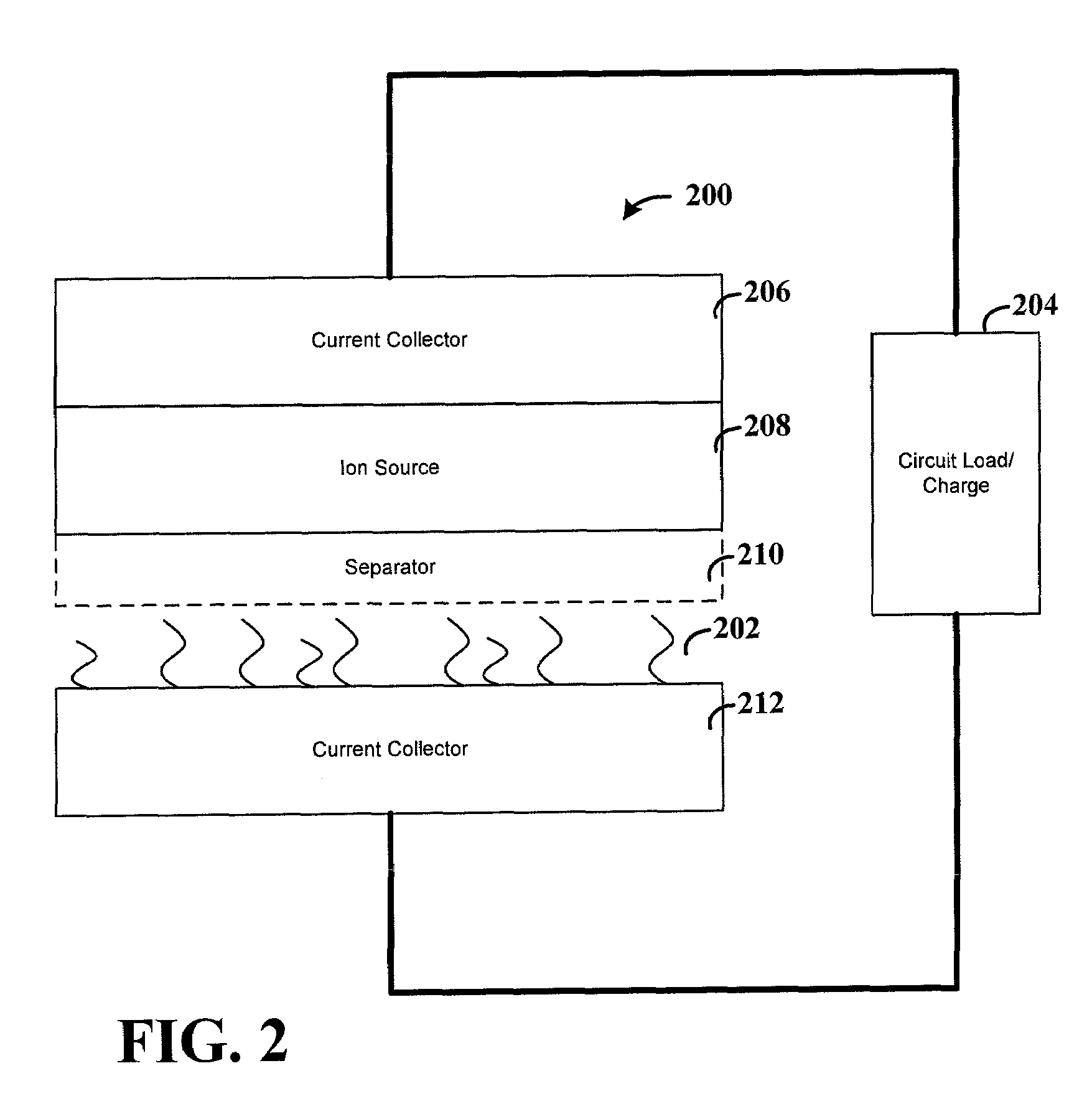
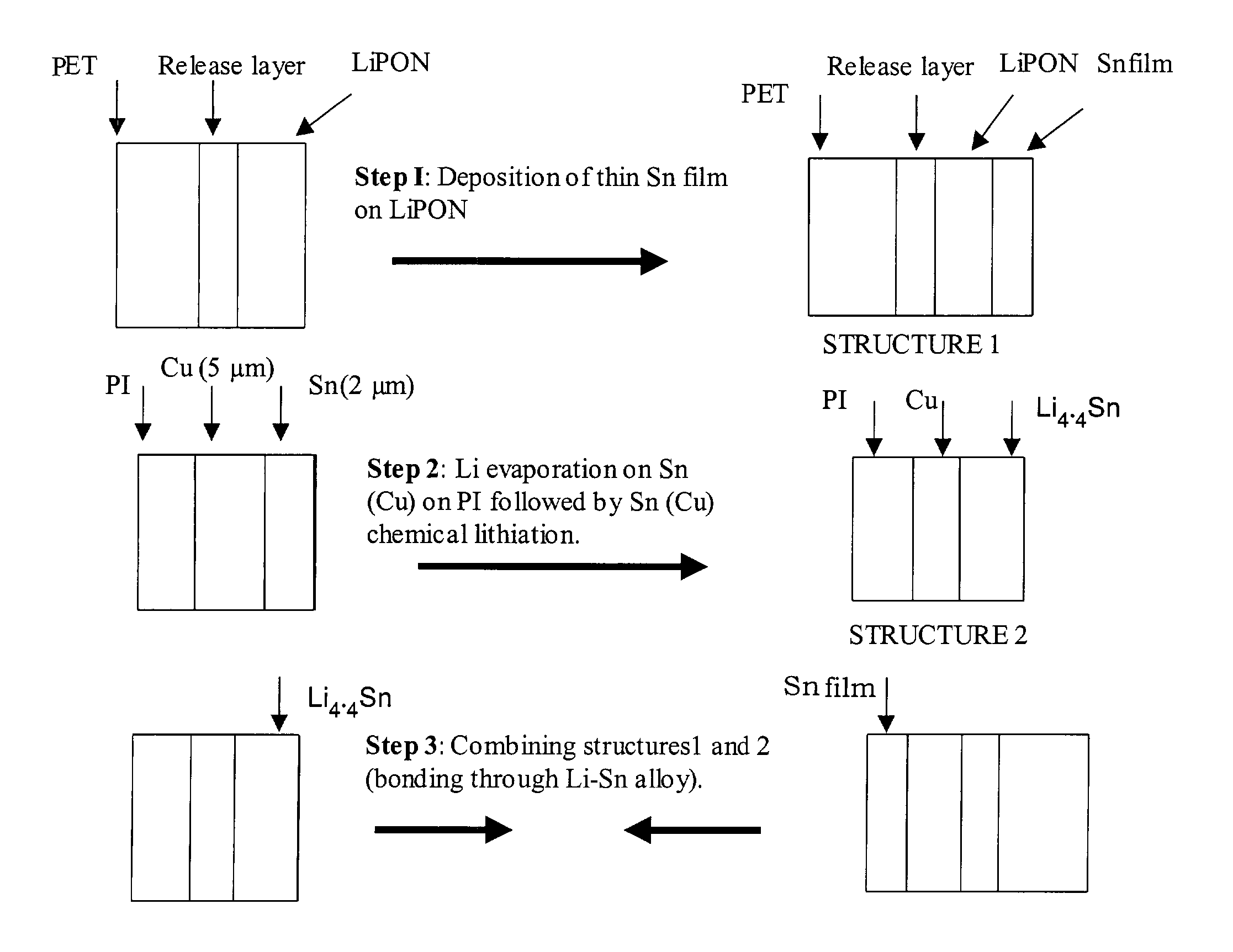
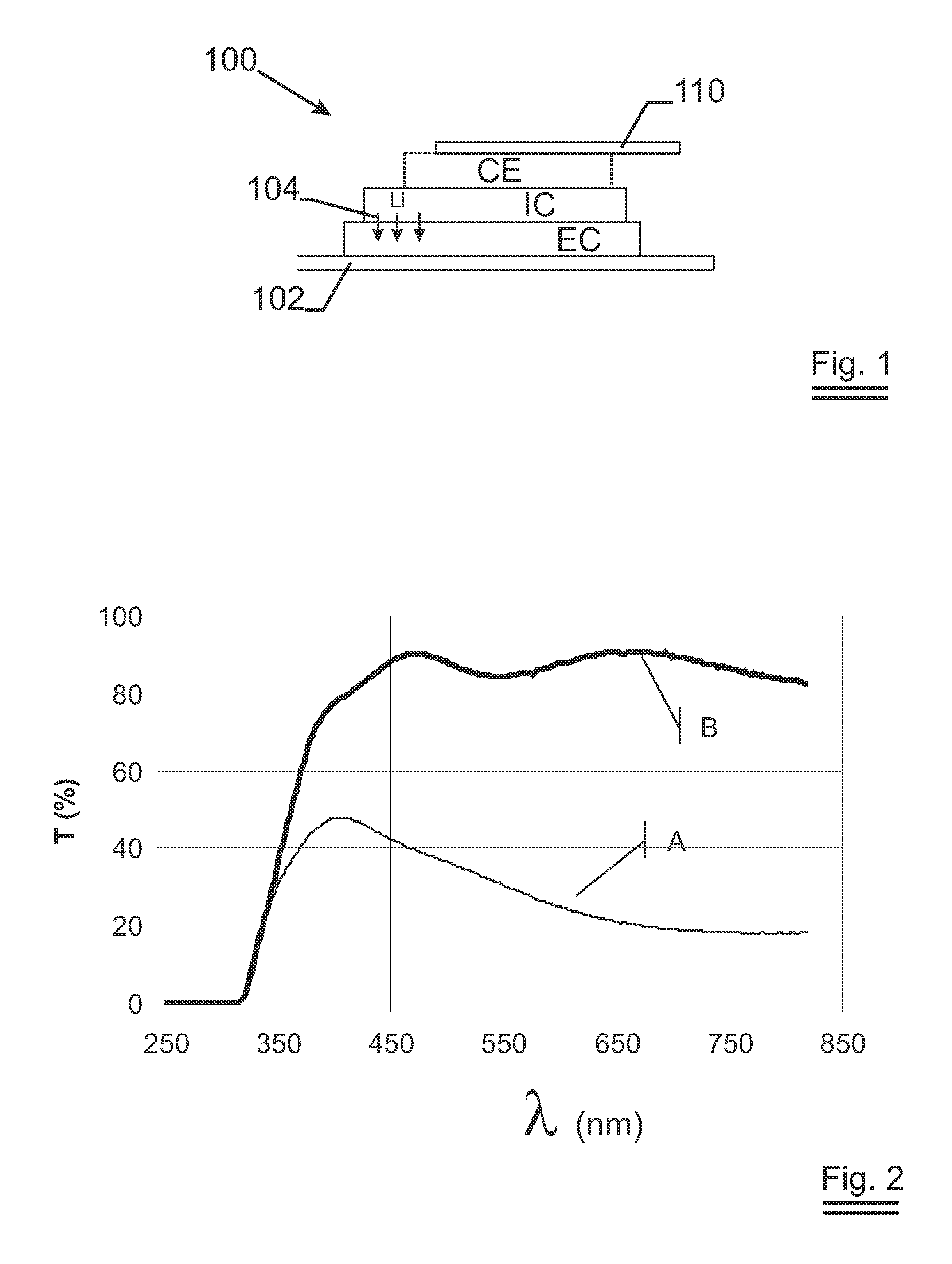
Lithium anodes for electrochemical cells
InactiveUS7247408B2Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
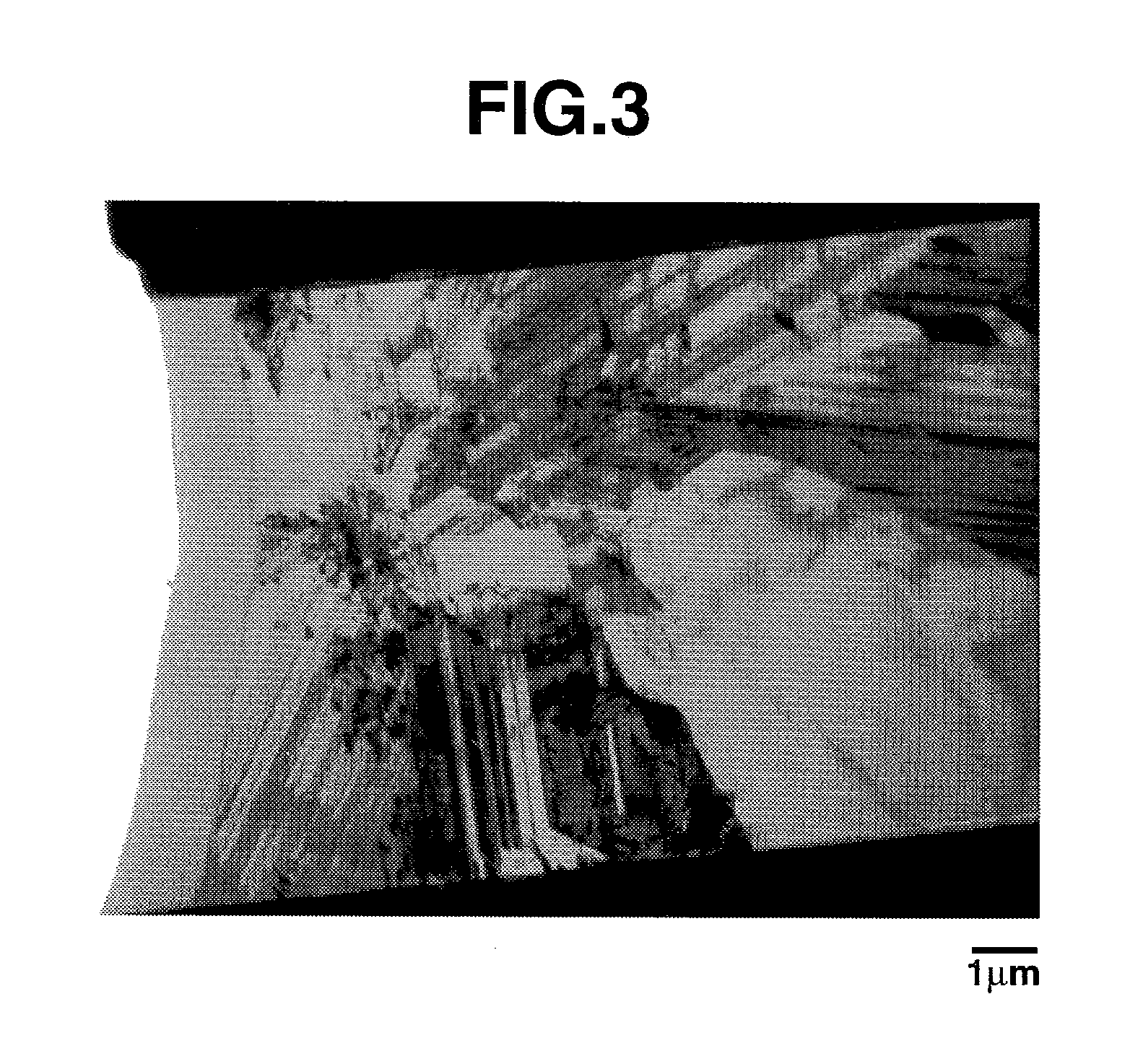
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
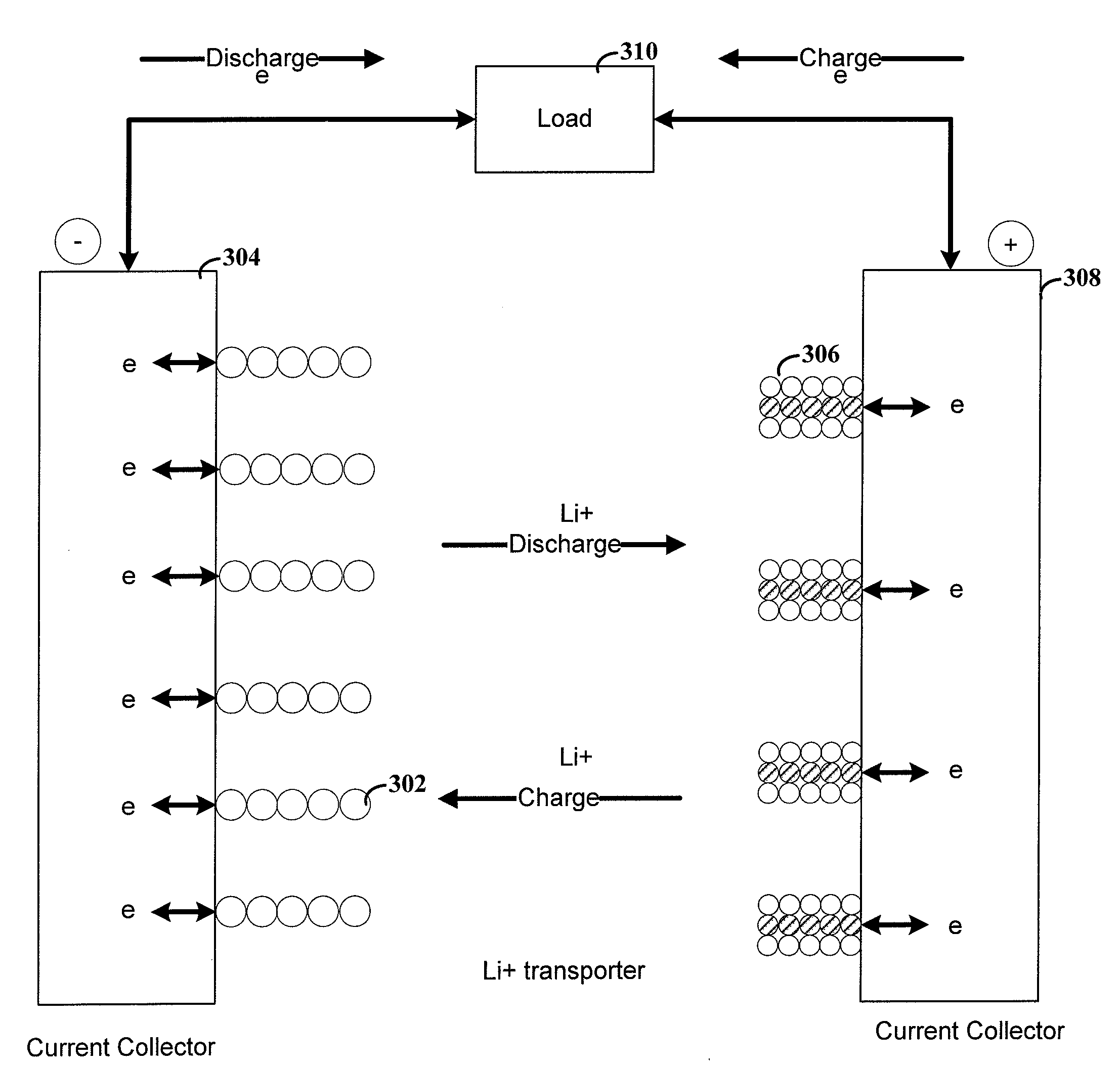
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6017651AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
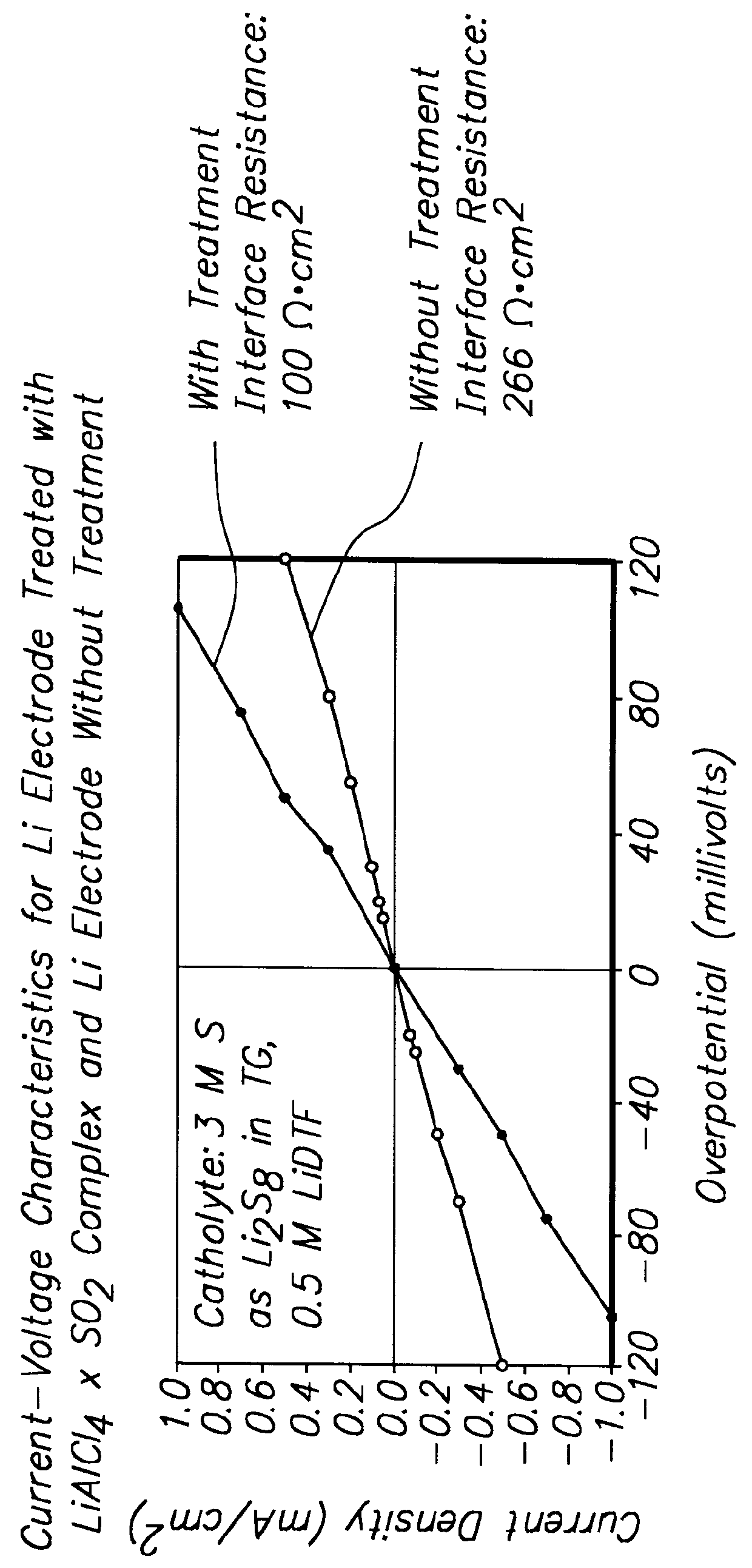
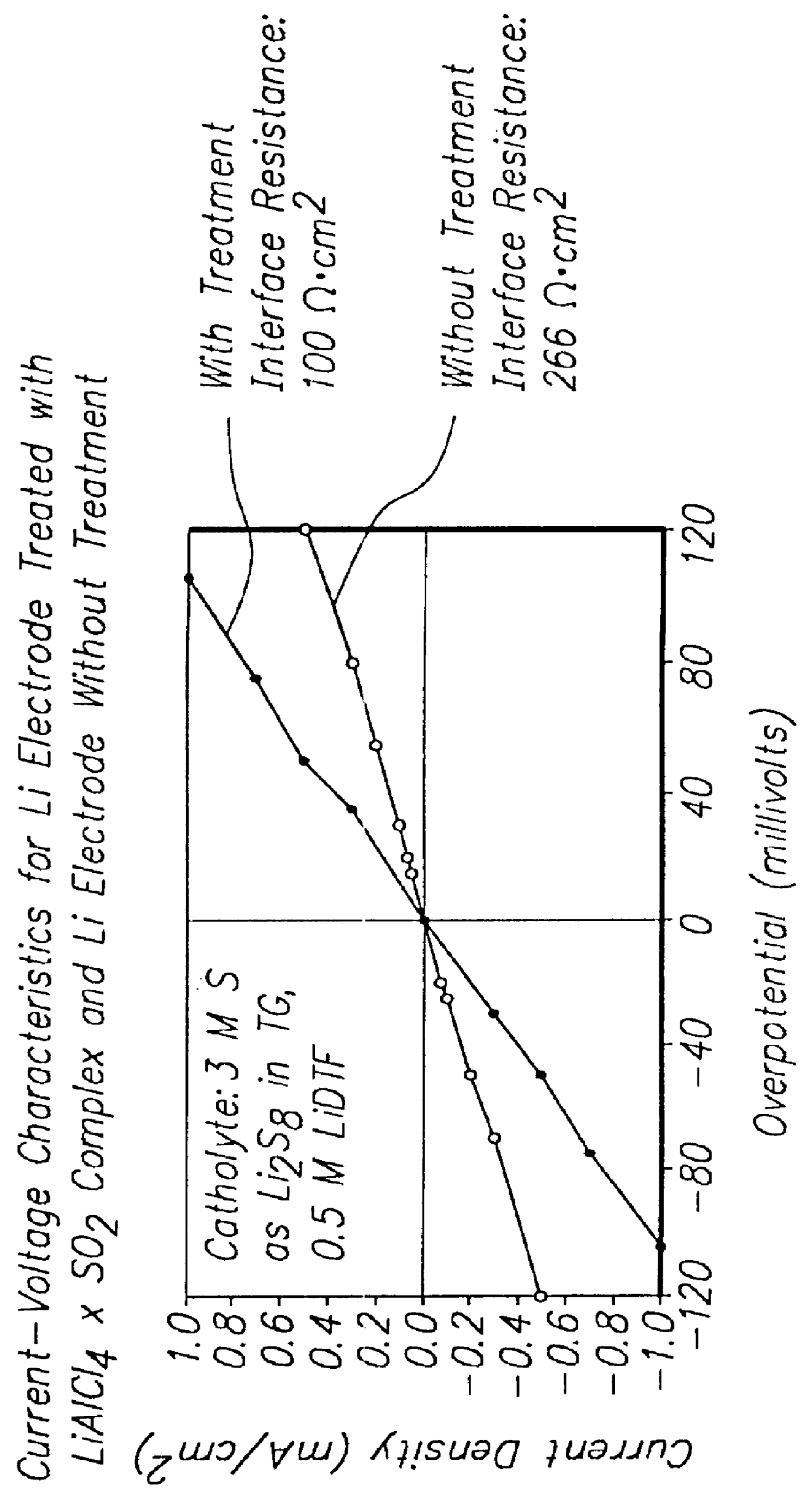
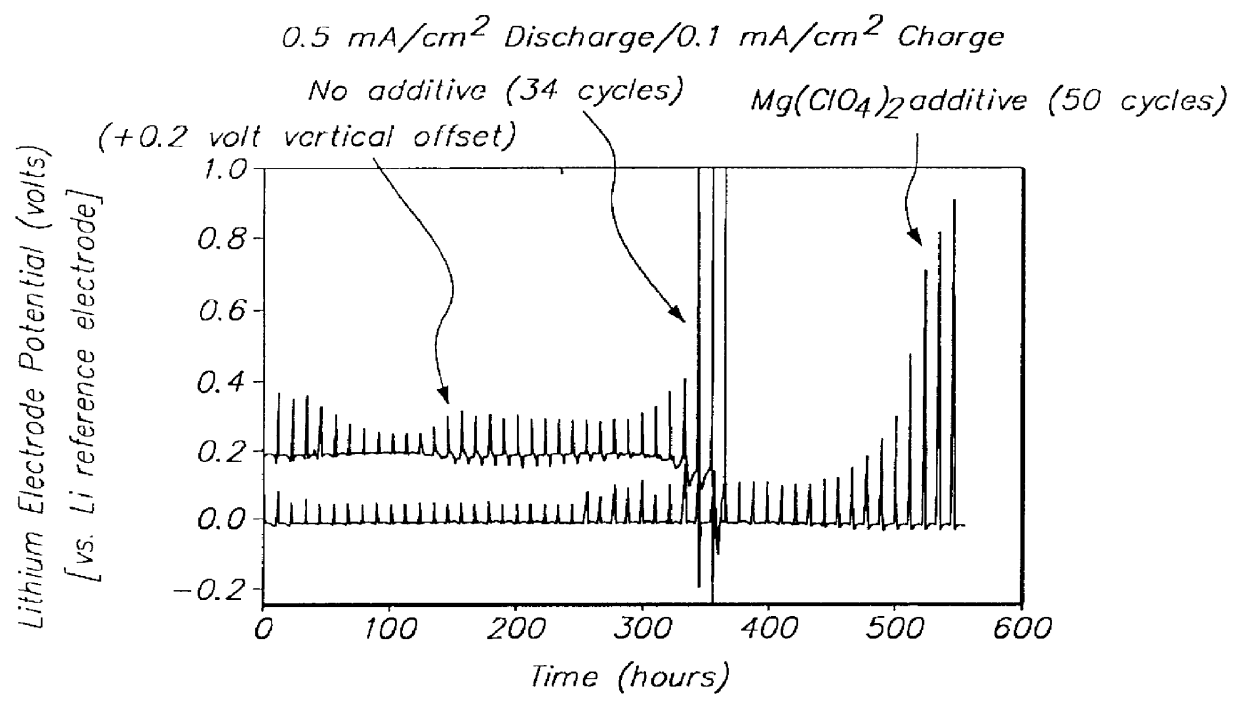
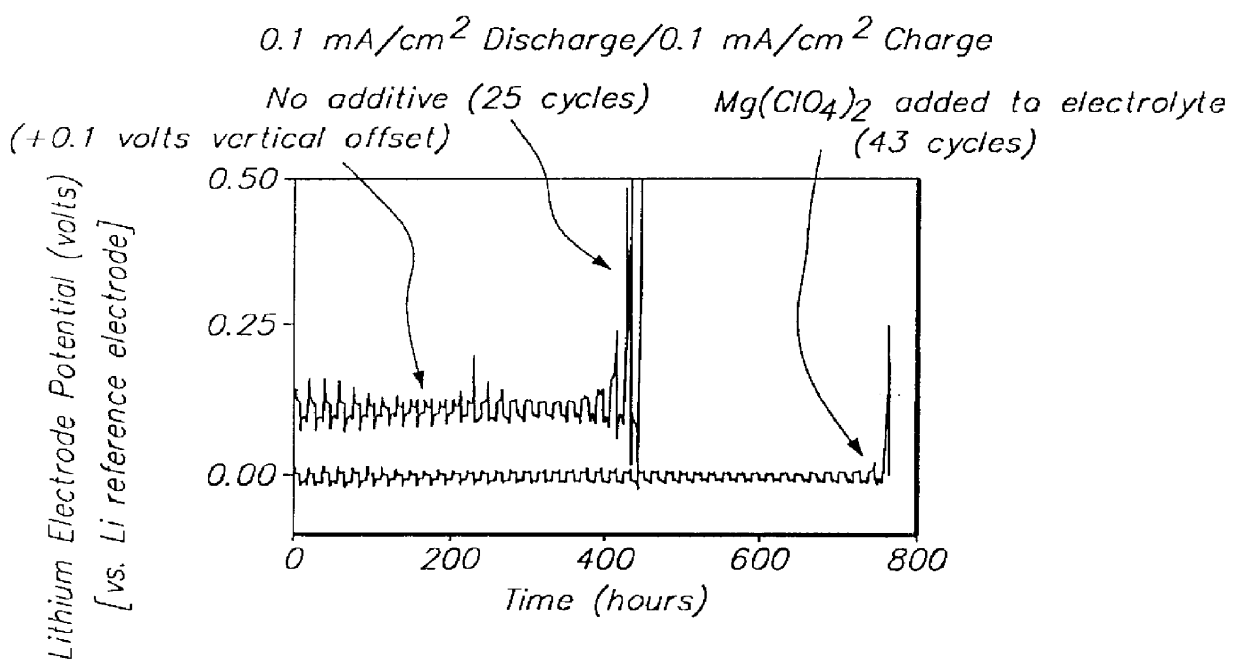
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
Lithium-ion rechargeable battery based on nanostructures
A nanowire-based Li-ion rechargeable battery having superior performance with little capacity fade for use in applications including consumer electronics and medical devices is made by incorporating nanowire construction of the cathode. The nanowire-based battery system includes a nanostructured high surface area cathode structure fabricated by electrodeposition using alumina nanopore templates.
Owner:ENABLE IPC
Method of depositing silicon on carbon materials and forming an anode for use in lithium ion batteries
ActiveUS20080261116A1Increase rangeMaterial nanotechnologyNanoinformaticsPhysical chemistryCarbon nanofiber
A method of modifying the surface of carbon materials such as vapor grown carbon nanofibers is provided in which silicon is deposited on vapor grown carbon nanofibers using a chemical vapor deposition process. The resulting silicon-carbon alloy may be used as an anode in a rechargeable lithium ion battery.
Owner:APPLIED SCI
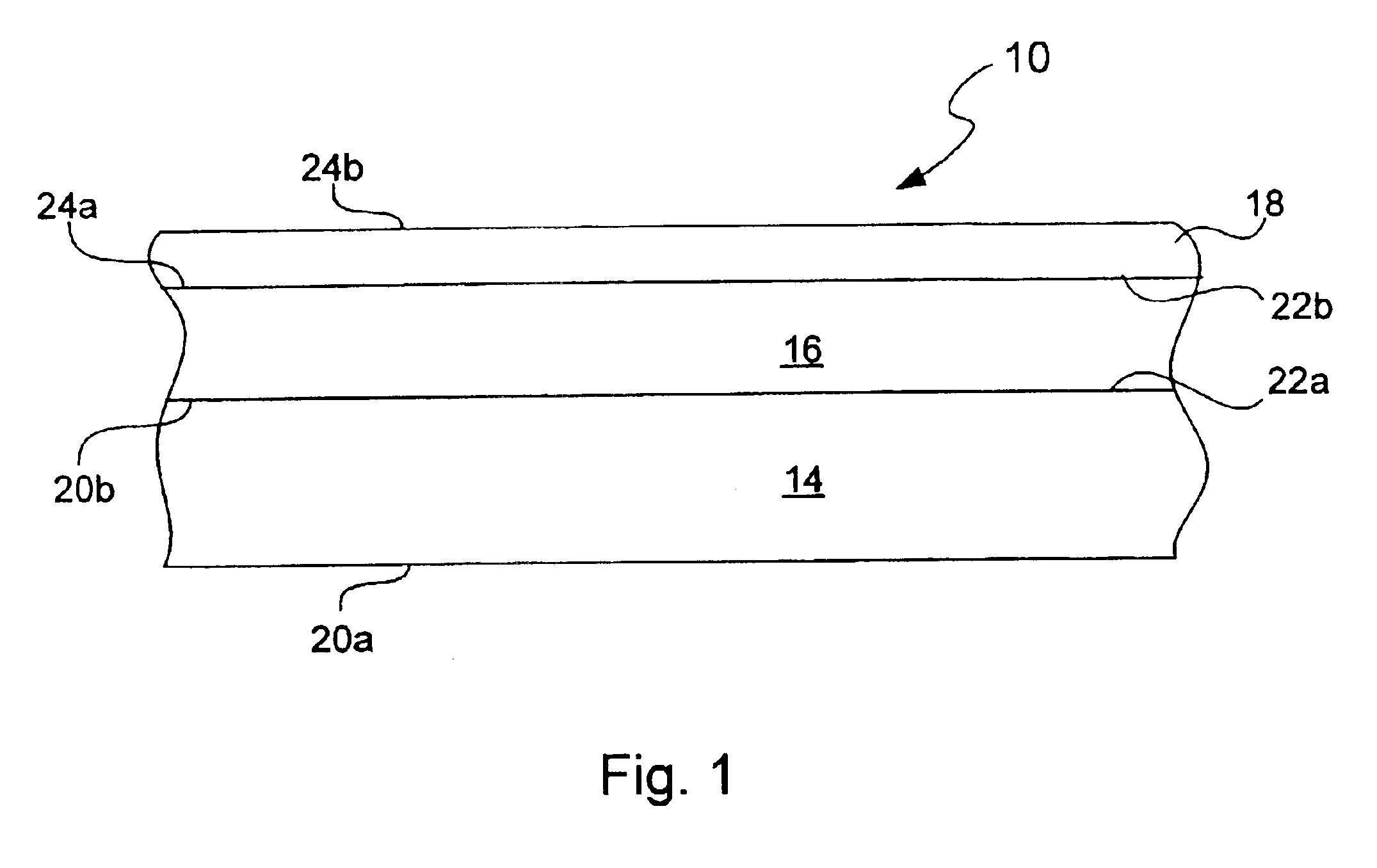
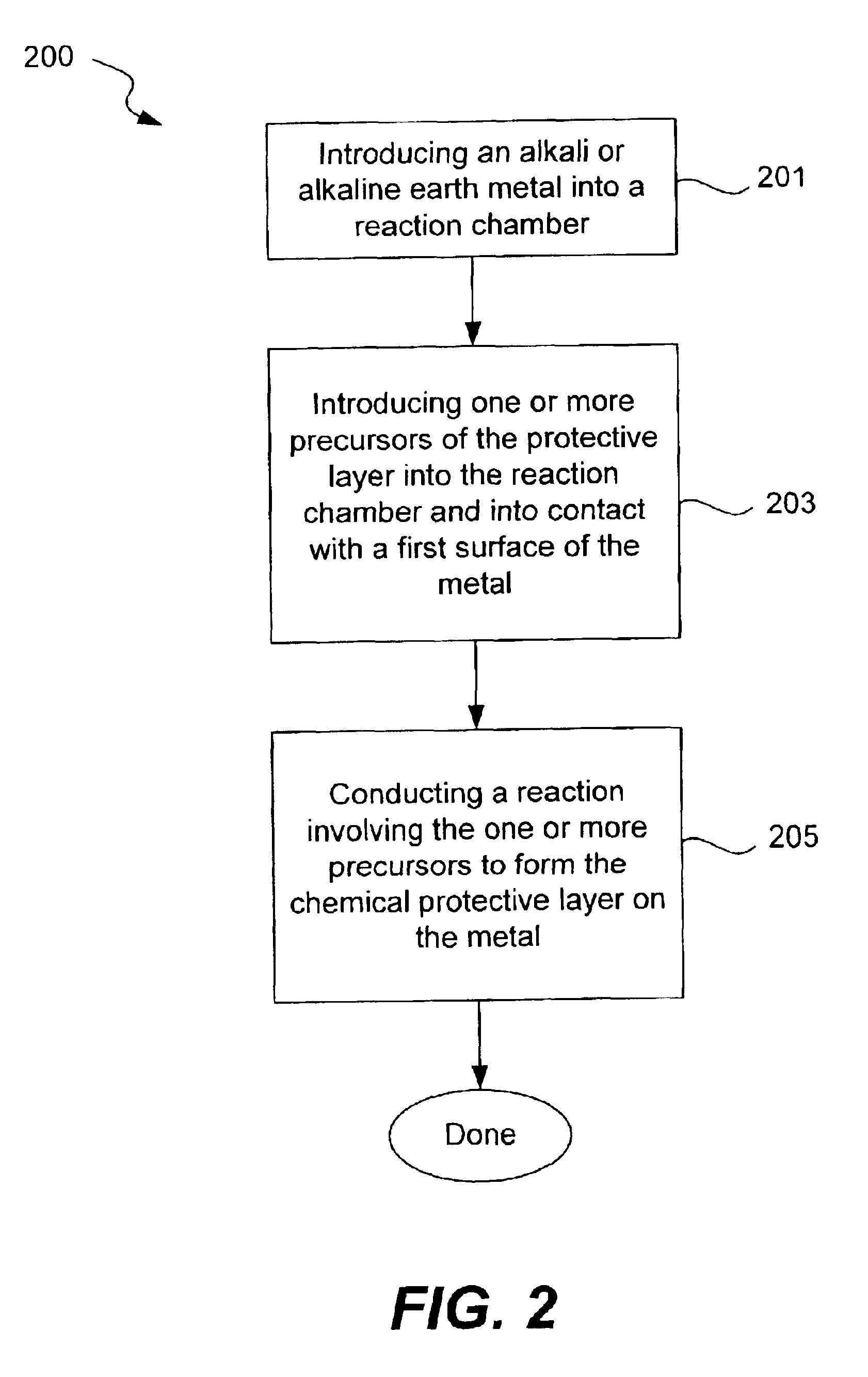
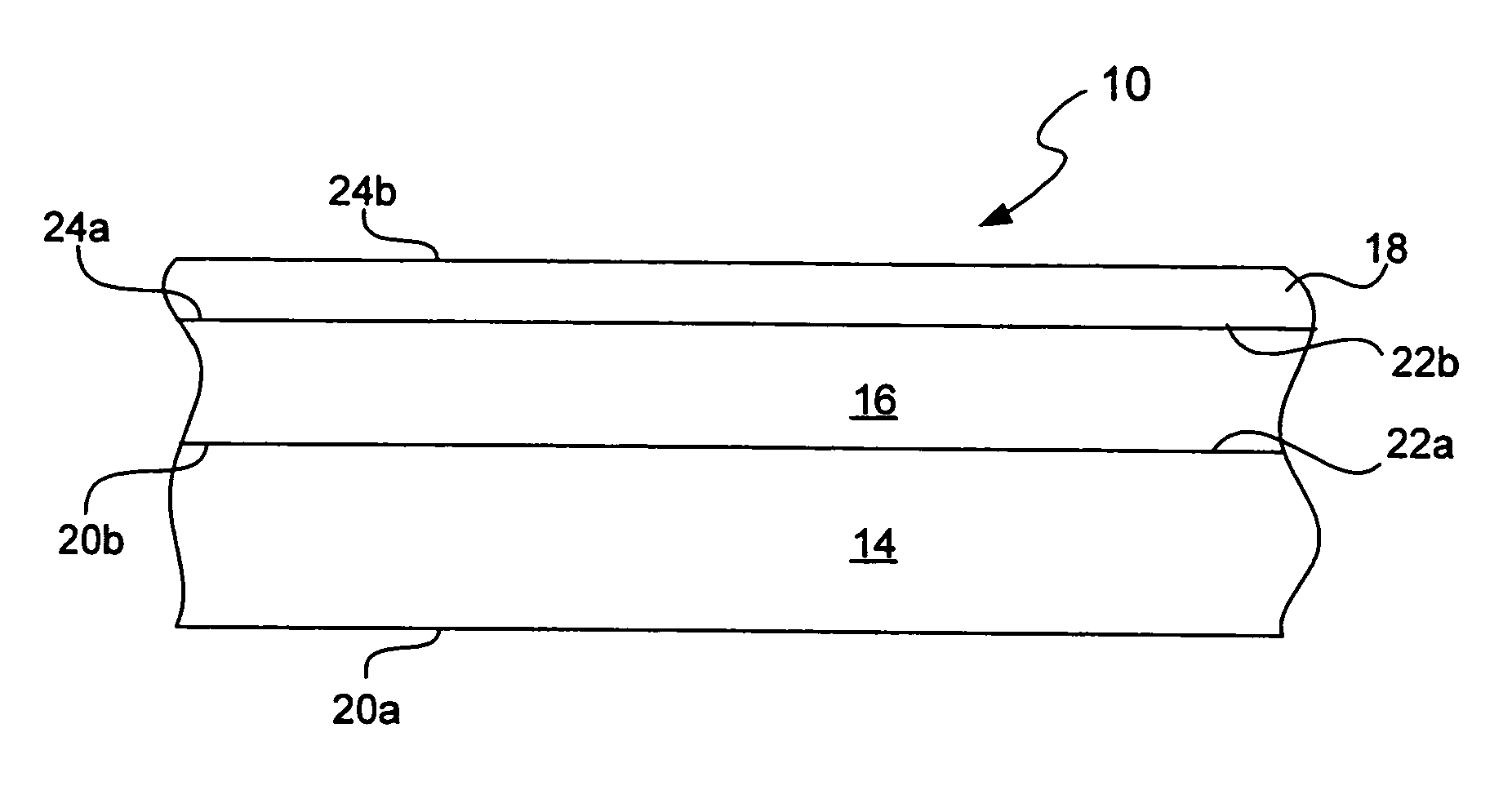
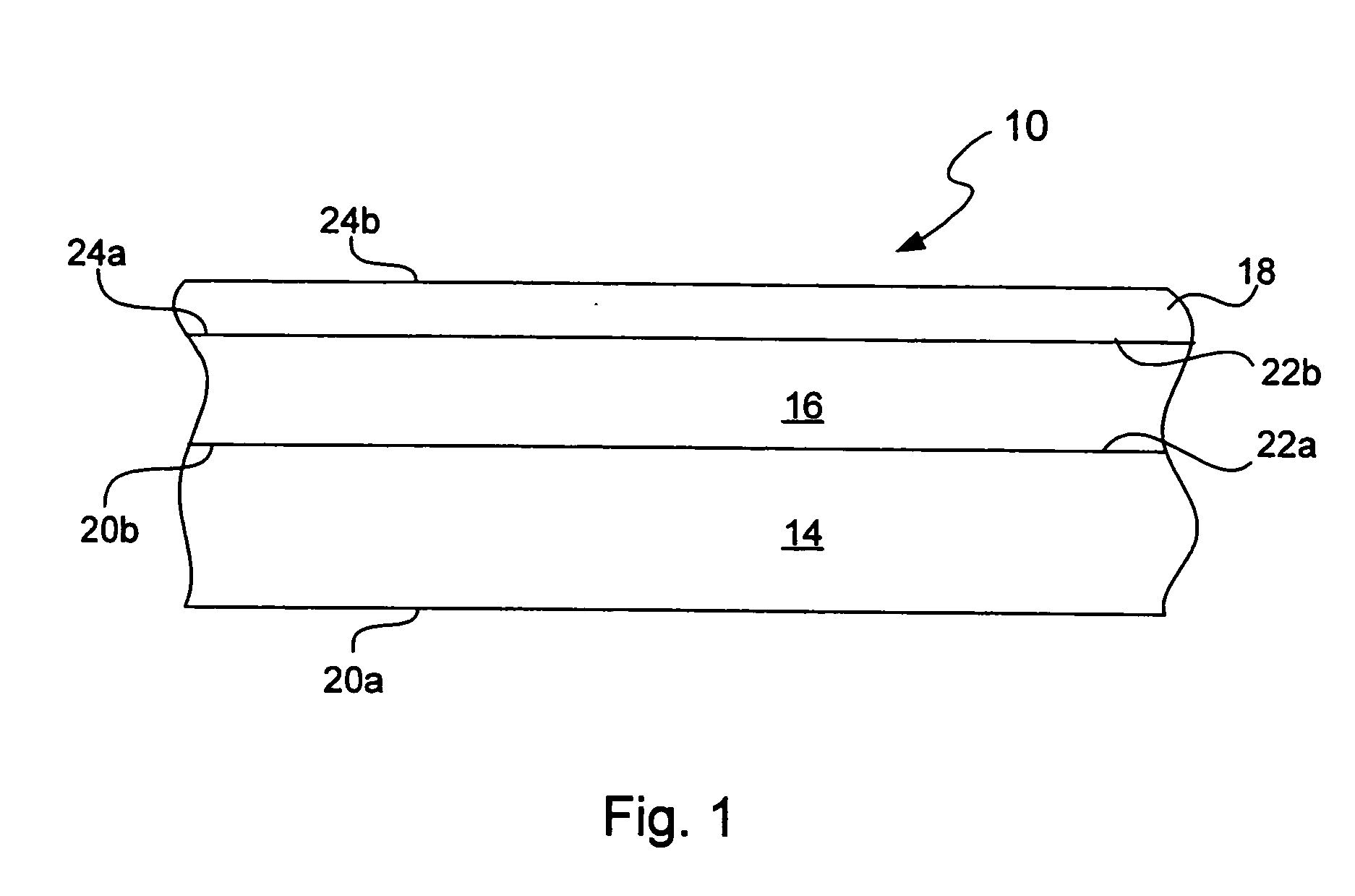
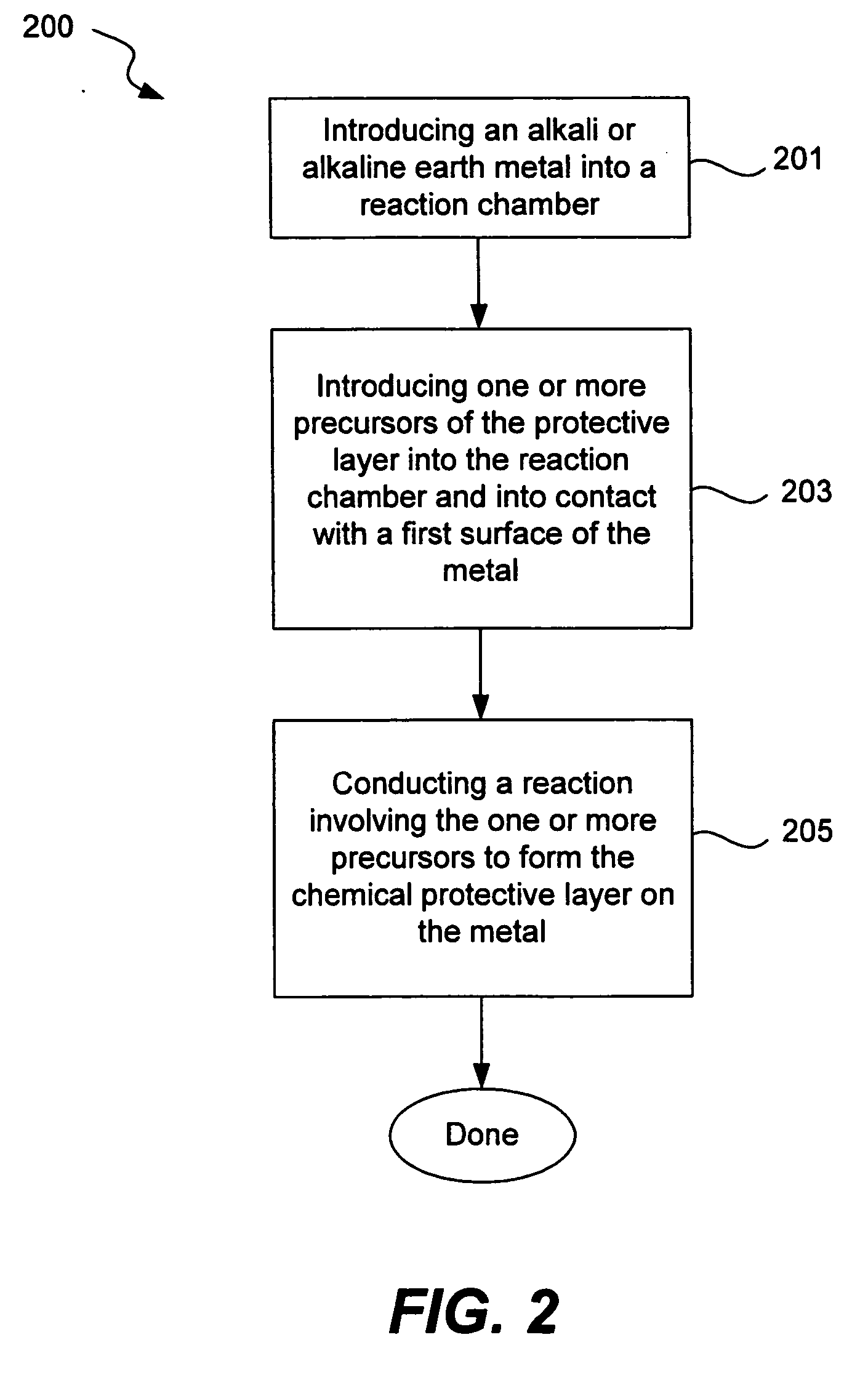
Chemical protection of a lithium surface
InactiveUS6911280B1Easy to produceSimple processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process in the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Chemical protection of a lithium surface
InactiveUS20050186469A1Easy to produceEasy to processElectrochemical processing of electrodesFinal product manufactureAlkaline earth metalLithium metal
Disclosed are compositions and methods for alleviating the problem of reaction of lithium or other alkali or alkaline earth metals with incompatible processing and operating environments by creating a ionically conductive chemical protective layer on the lithium or other reactive metal surface. Such a chemically produced surface layer can protect lithium metal from reacting with oxygen, nitrogen or moisture in ambient atmosphere thereby allowing the lithium material to be handled outside of a controlled atmosphere, such as a dry room. Production processes involving lithium are thereby very considerably simplified. One example of such a process is the processing of lithium to form negative electrodes for lithium metal batteries.
Owner:POLYPLUS BATTERY CO INC
Method for preparing electrode material for lithium battery
InactiveUS6887511B1Improve adhesionReduce expansionElectrode carriers/collectorsVacuum evaporation coatingAmorphous siliconOptoelectronics
Owner:SANYO ELECTRIC CO LTD
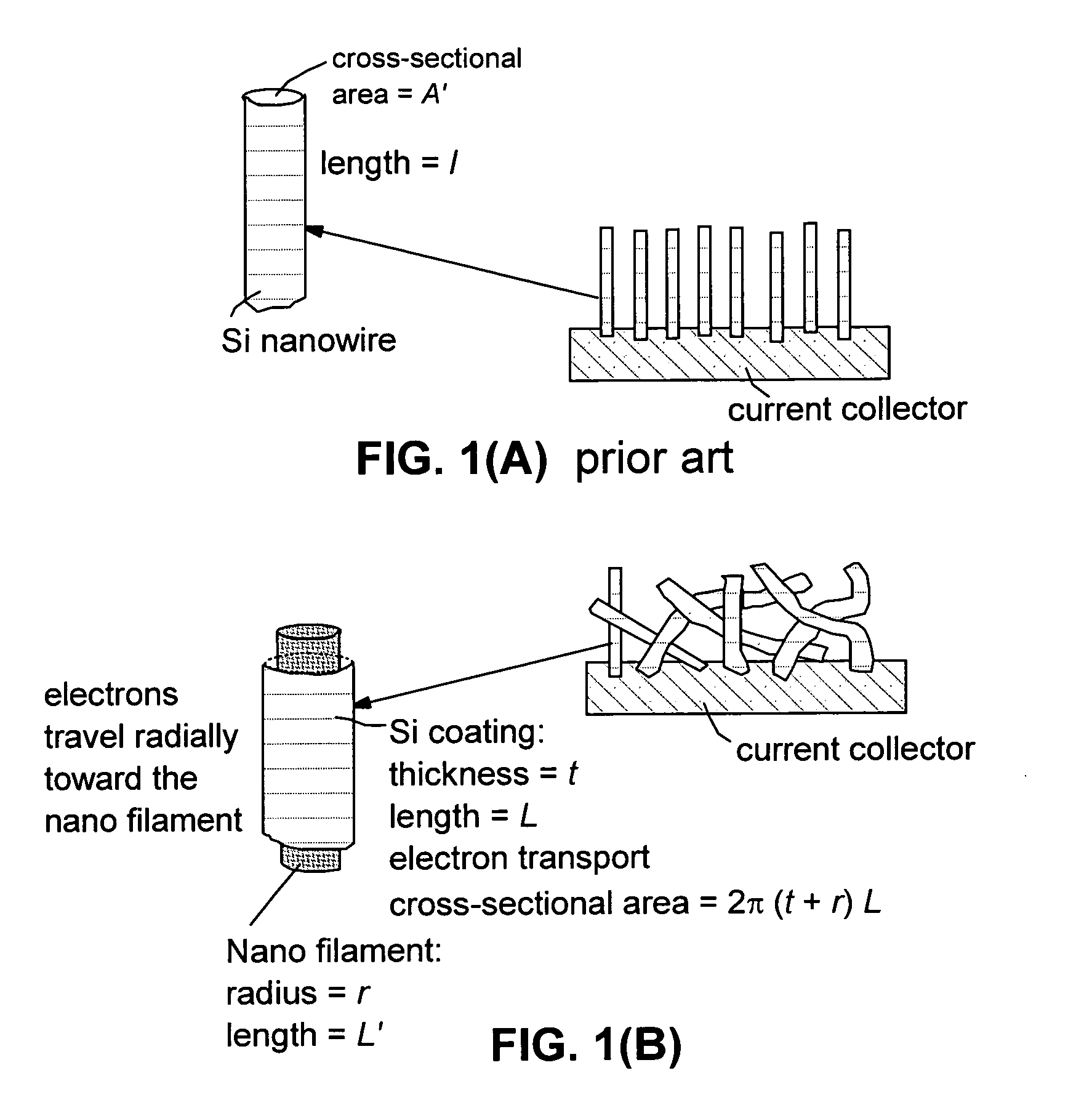
Hybrid nano-filament anode compositions for lithium ion batteries
ActiveUS20090169996A1Superior multiple-cycle behaviorImprove cycle lifeElectrochemical processing of electrodesElectrode thermal treatmentLithium-ion batteryNanometre
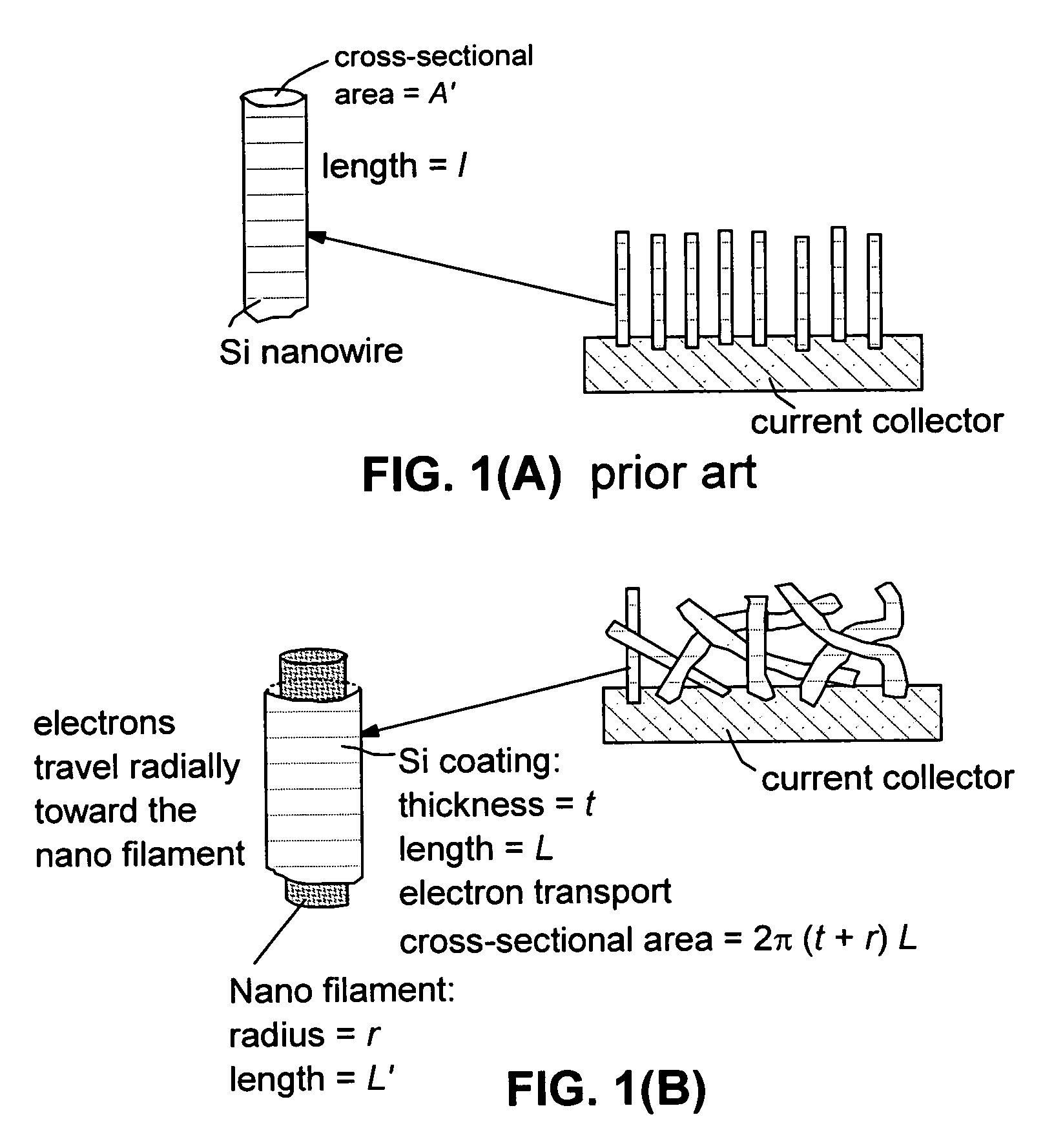
This invention provides a hybrid nano-filament composition for use as an electrochemical cell electrode. The composition comprises: (a) an aggregate of nanometer-scaled, electrically conductive filaments that are substantially interconnected, intersected, or percolated to form a porous, electrically conductive filament network comprising substantially interconnected pores, wherein the filaments have an elongate dimension and a first transverse dimension with the first transverse dimension being less than 500 nm (preferably less than 100 nm) and an aspect ratio of the elongate dimension to the first transverse dimension greater than 10; and (b) micron- or nanometer-scaled coating that is deposited on a surface of the filaments, wherein the coating comprises an anode active material capable of absorbing and desorbing lithium ions and the coating has a thickness less than 20 μm (preferably less than 1 μm). Also provided is a lithium ion battery comprising such an electrode as an anode. The battery exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Non-aqueous electrolyte secondary battery, negative electrode material, and making method
ActiveUS20090239151A1Improve cycle performanceImprove efficiencyNon-aqueous electrolyte accumulatorsActive material electrodesSilicon oxideSilicon particle
A negative electrode material comprising an active material and 1-20 wt % of a polyimide resin binder is suitable for use in non-aqueous electrolyte secondary batteries. The active material comprises silicon oxide particles and 1-50 wt % of silicon particles. The negative electrode exhibits improved cycle performance while maintaining the high battery capacity and low volume expansion of silicon oxide. The non-aqueous electrolyte secondary battery has a high initial efficiency and maintains improved performance and efficiency over repeated charge / discharge cycles by virtue of mitigated volumetric changes during charge / discharge cycles.
Owner:SHIN ETSU CHEM IND CO LTD
Lithium anodes for electrochemical cells
InactiveUS20060222954A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Electrochemical apparatus with barrier layer protected substrate
ActiveUS20060286448A1Inhibited DiffusionEfficient separationFinal product manufactureElectrode carriers/collectorsElectrochemistrySilicon
The present invention relates to apparatus, compositions and methods of fabricating high performance thin-film batteries on metallic substrates, polymeric substrates, or doped or undoped silicon substrates by fabricating an appropriate barrier layer composed, for example, of barrier sublayers between the substrate and the battery part of the present invention thereby separating these two parts chemically during the entire battery fabrication process as well as during any operation and storage of the electrochemical apparatus during its entire lifetime. In a preferred embodiment of the present invention thin-film batteries fabricated onto a thin, flexible stainless steel foil substrate using an appropriate barrier layer that is composed of barrier sublayers have uncompromised electrochemical performance compared to thin-film batteries fabricated onto ceramic substrates when using a 700° C. post-deposition anneal process for a LiCoO2 positive cathode.
Owner:SAPURAST RES
Nanowire Battery Methods and Arrangements
ActiveUS20090042102A1High energy capacityNanostructure manufactureFinal product manufactureNanowireNanowire battery
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Electrode material for electrochemcial device, method for producing the same, electrode using the electrode material, and electrochemical device using the electrode material
An electrode material of the present invention includes a plurality of particles capable of absorbing and desorbing lithium, and a plurality of nanowires capable of absorbing and desorbing lithium. The particles and the nanowires include silicon atoms. The plurality of nanowires are entangled with each other to form a network, and the network is in contact with at least two of the plurality of particles.
Owner:PANASONIC CORP
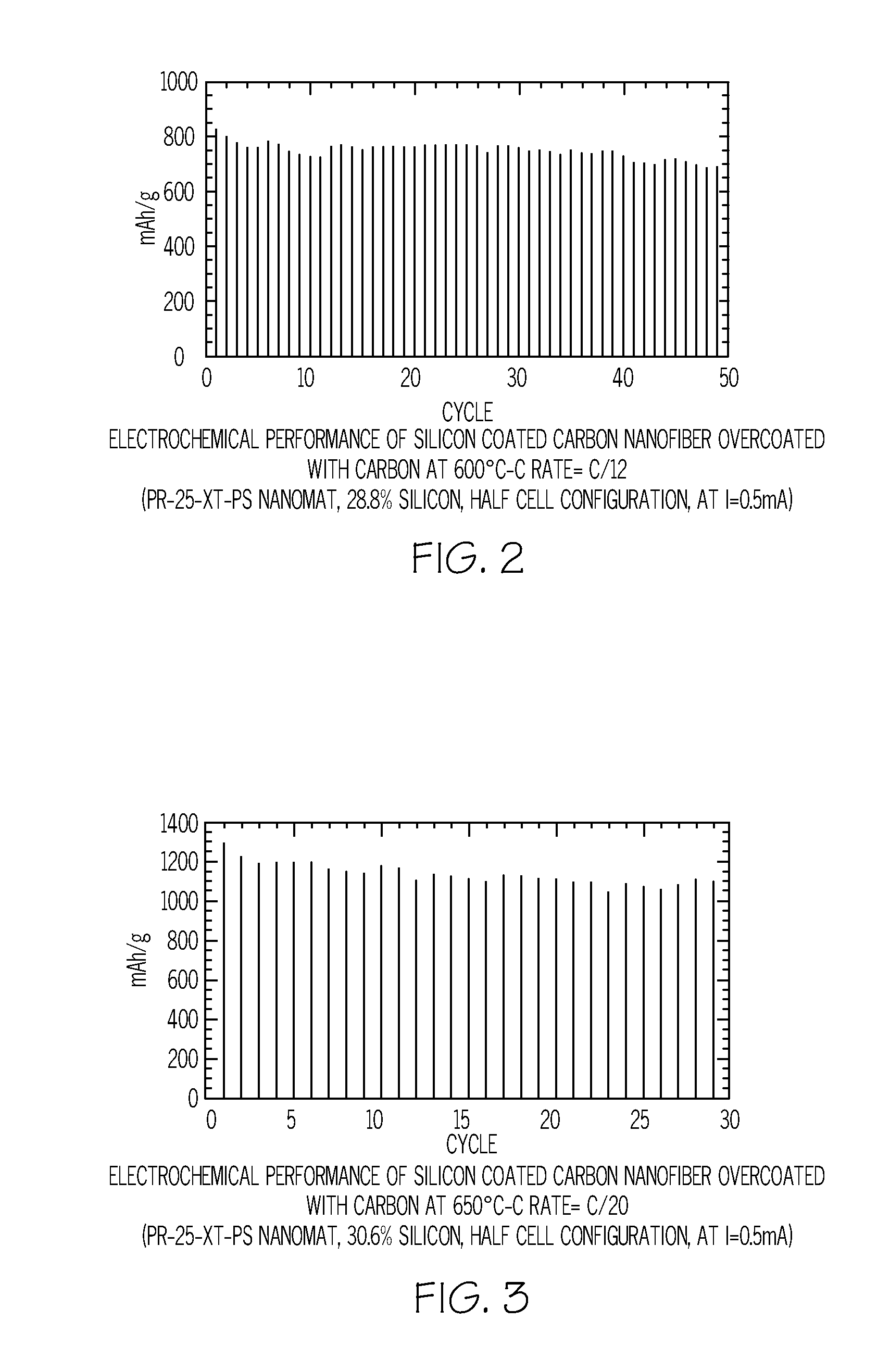
Method of depositing silicon on carbon nanomaterials
InactiveUS20120264020A1Increase surface areaHigh pore volumeMaterial nanotechnologyLiquid surface applicatorsCarbon coatingGas phase
A method of depositing silicon on carbon nanomaterials such as vapor grown carbon nanofibers, nanomats, or nanofiber powder is provided. The method includes flowing a silicon-containing precursor gas in contact with the carbon nanomaterial such that silicon is deposited on the exterior surface and within the hollow core of the carbon nanomaterials. A protective carbon coating may be deposited on the silicon-coated nanomaterials. The resulting nanocomposite materials may be used as anodes in lithium ion batteries.
Owner:APPLIED SCI
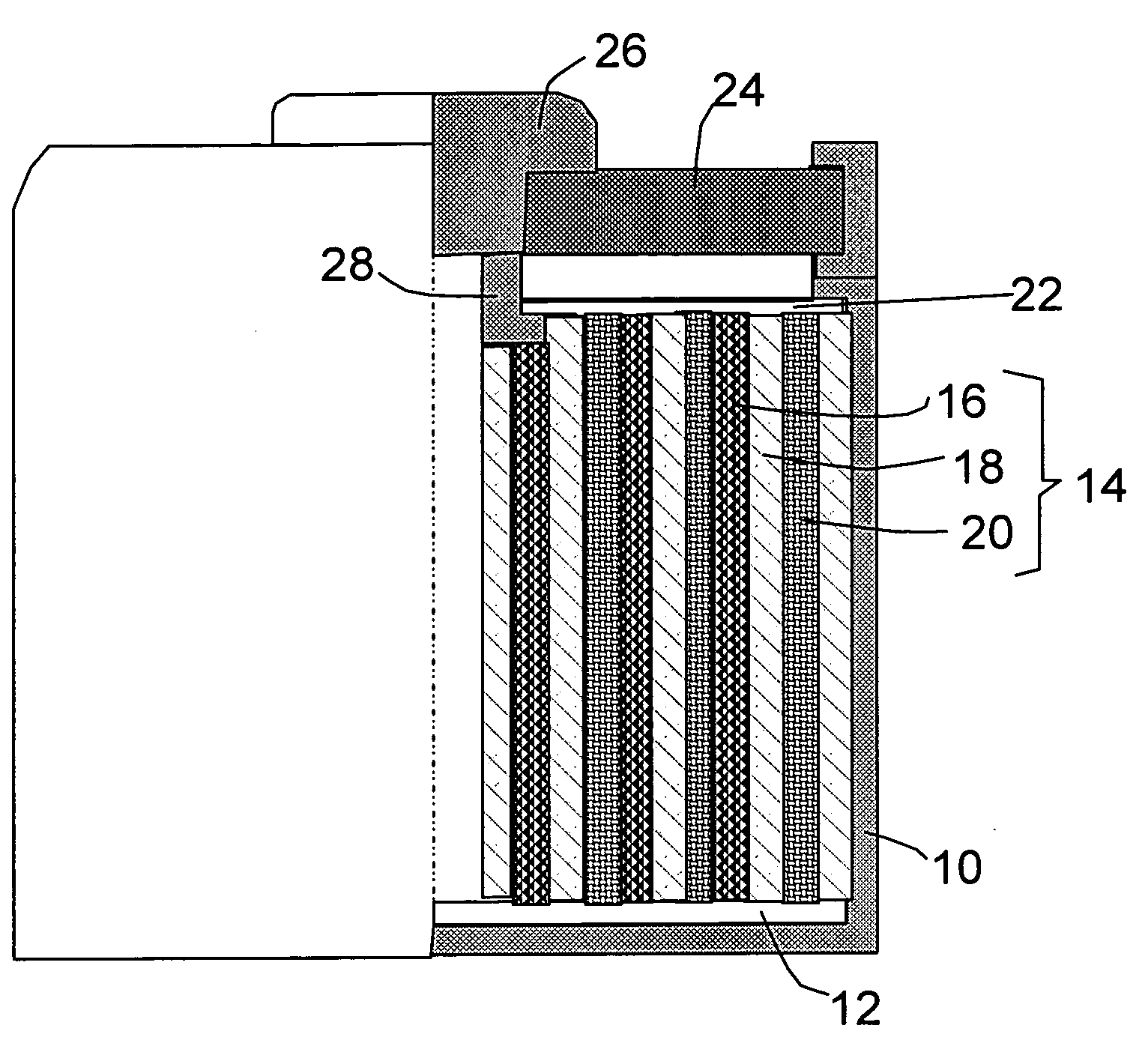
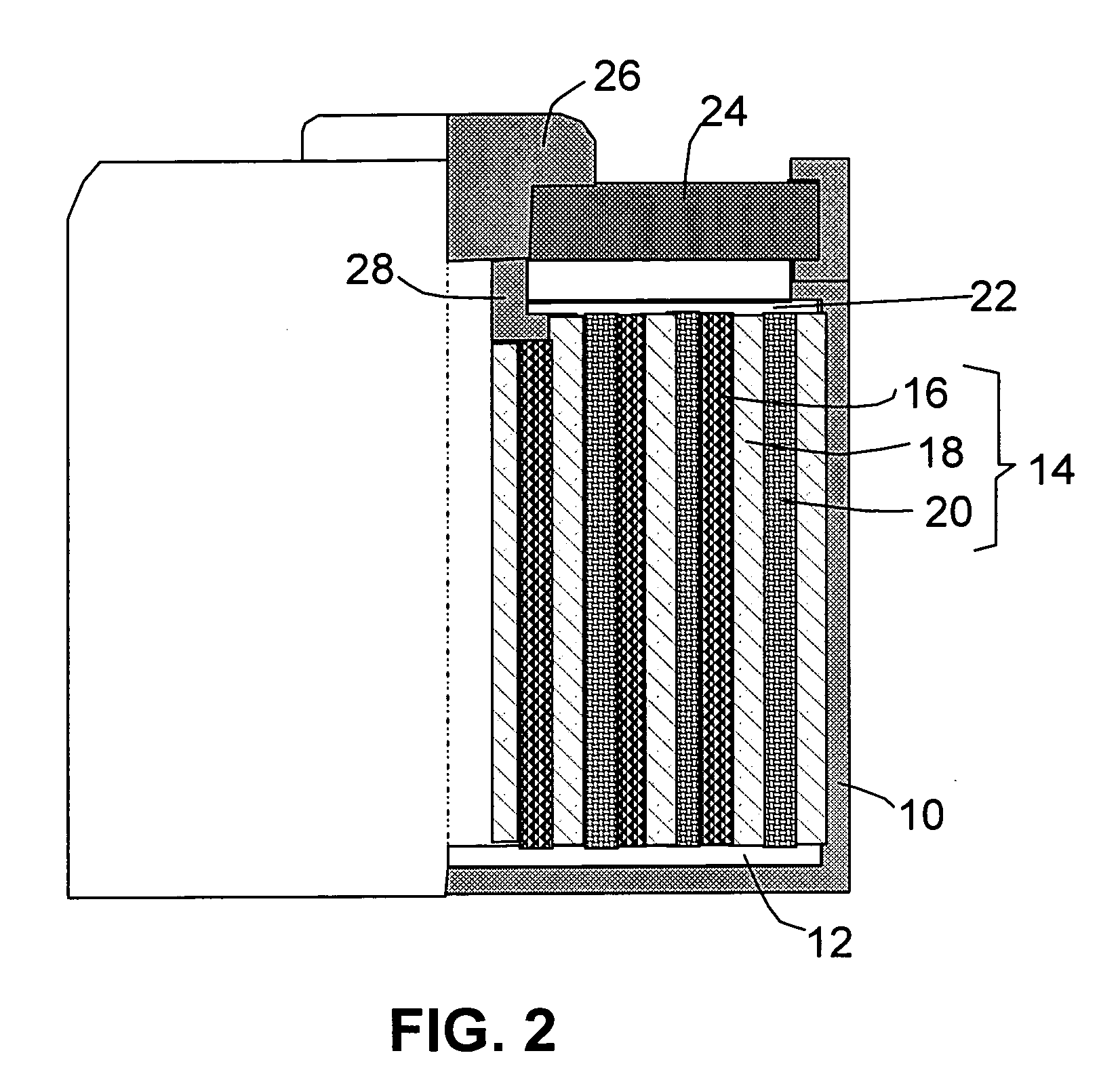
Thin film electrochemical energy storage device with three-dimensional anodic structure
InactiveUS20100216026A1Final product manufactureElectrode carriers/collectorsPorosityMicro structure
A method for forming a battery from via thin-film deposition processes is disclosed. A mesoporous carbon material is deposited onto a surface of a conductive substrate that has high surface area, conductive micro-structures formed thereon. A porous, dielectric separator layer is then deposited on the layer of mesoporous carbon material to form a half cell of an energy storage device. The mesoporous carbon material is made up of CVD-deposited carbon fullerene “onions” and carbon nano-tubes, and has a high porosity capable of retaining lithium ions in concentrations useful for storing significant quantities of electrical energy. Embodiments of the invention further provide for the formation of an electrode having a high surface area conductive region that is useful in a battery structure. In one configuration the electrode has a high surface area conductive region comprising a porous dendritic structure that can be formed by electroplating, physical vapor deposition, chemical vapor deposition, thermal spraying, and / or electroless plating techniques.
Owner:APPLIED MATERIALS INC
Method of producing hybrid nano-filament electrodes for lithium metal or lithium ion batteries
ActiveUS20090169725A1High reversible capacityLower internal resistanceElectrochemical processing of electrodesElectrode thermal treatmentChemical LinkageFiber
Disclosed is a method of producing a hybrid nano-filament composition for use in a lithium battery electrode. The method comprises: (a) providing an aggregate of nanometer-scaled, electrically conductive filaments that are substantially interconnected, intersected, physically contacted, or chemically bonded to form a porous network of electrically conductive filaments, wherein the filaments comprise electro-spun nano-fibers that have a diameter less than 500 nm (preferably less than 100 nm); and (b) depositing micron- or nanometer-scaled coating onto a surface of the electro-spun nano-fibers, wherein the coating comprises an electro-active material capable of absorbing and desorbing lithium ions and the coating has a thickness less than 10 μm (preferably less than 1 μm). The same method can be followed to produce an anode or a cathode. The battery featuring an anode or cathode made with this method exhibits an exceptionally high specific capacity, an excellent reversible capacity, and a long cycle life.
Owner:GLOBAL GRAPHENE GRP INC
Lithium secondary battery
InactiveUS20070031733A1Negative currentEasy dischargeFinal product manufactureElectrode carriers/collectorsLithiumSilicon
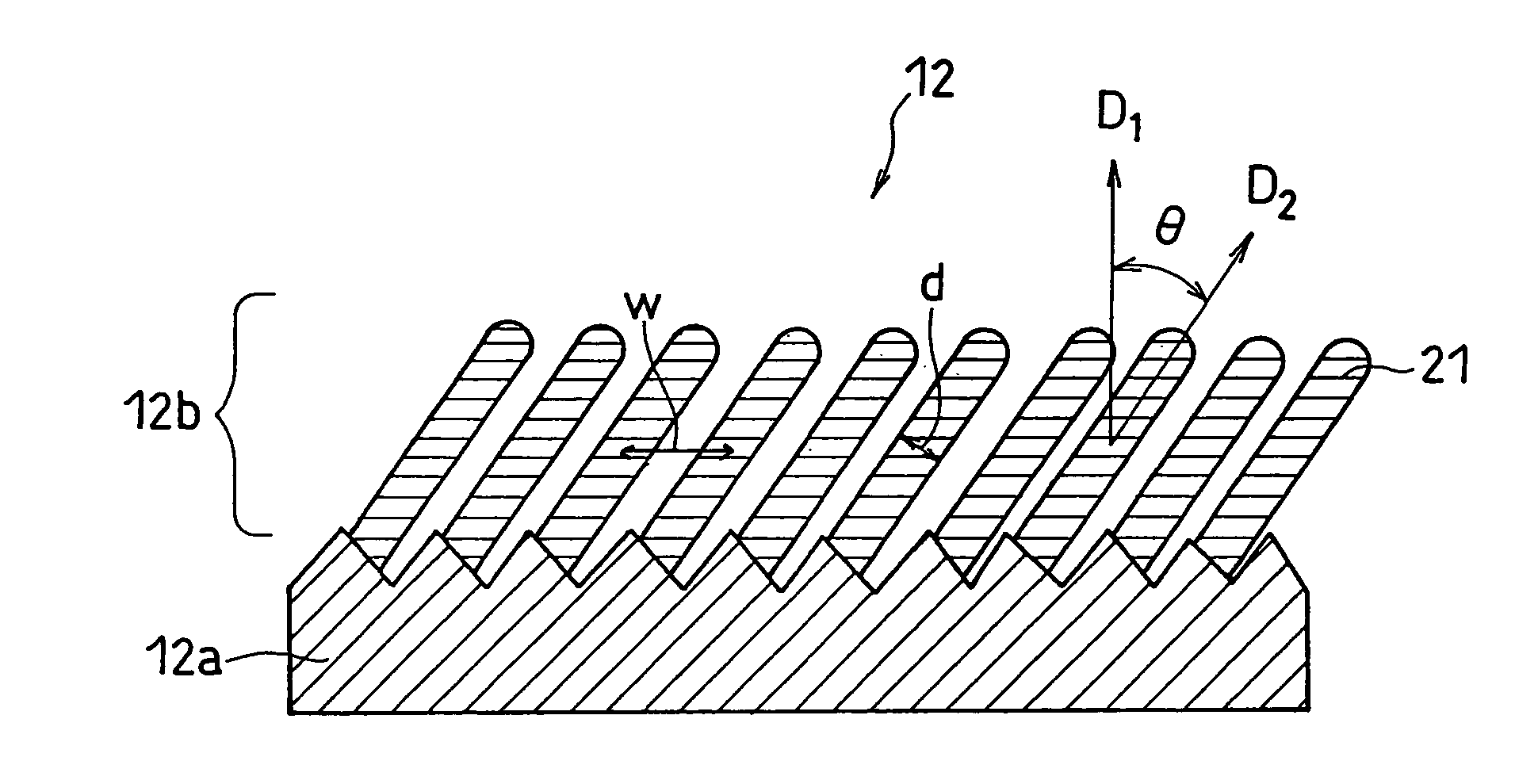
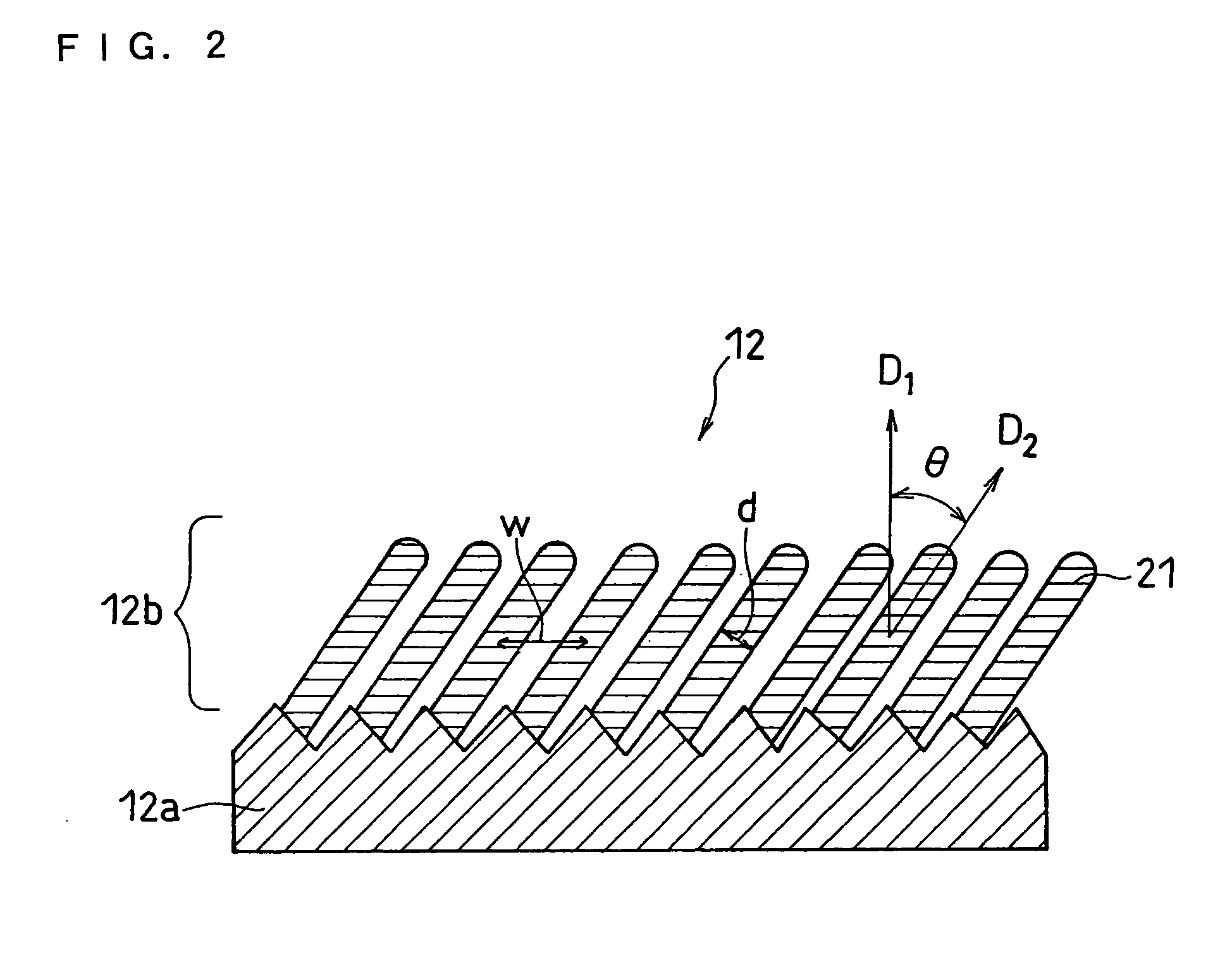
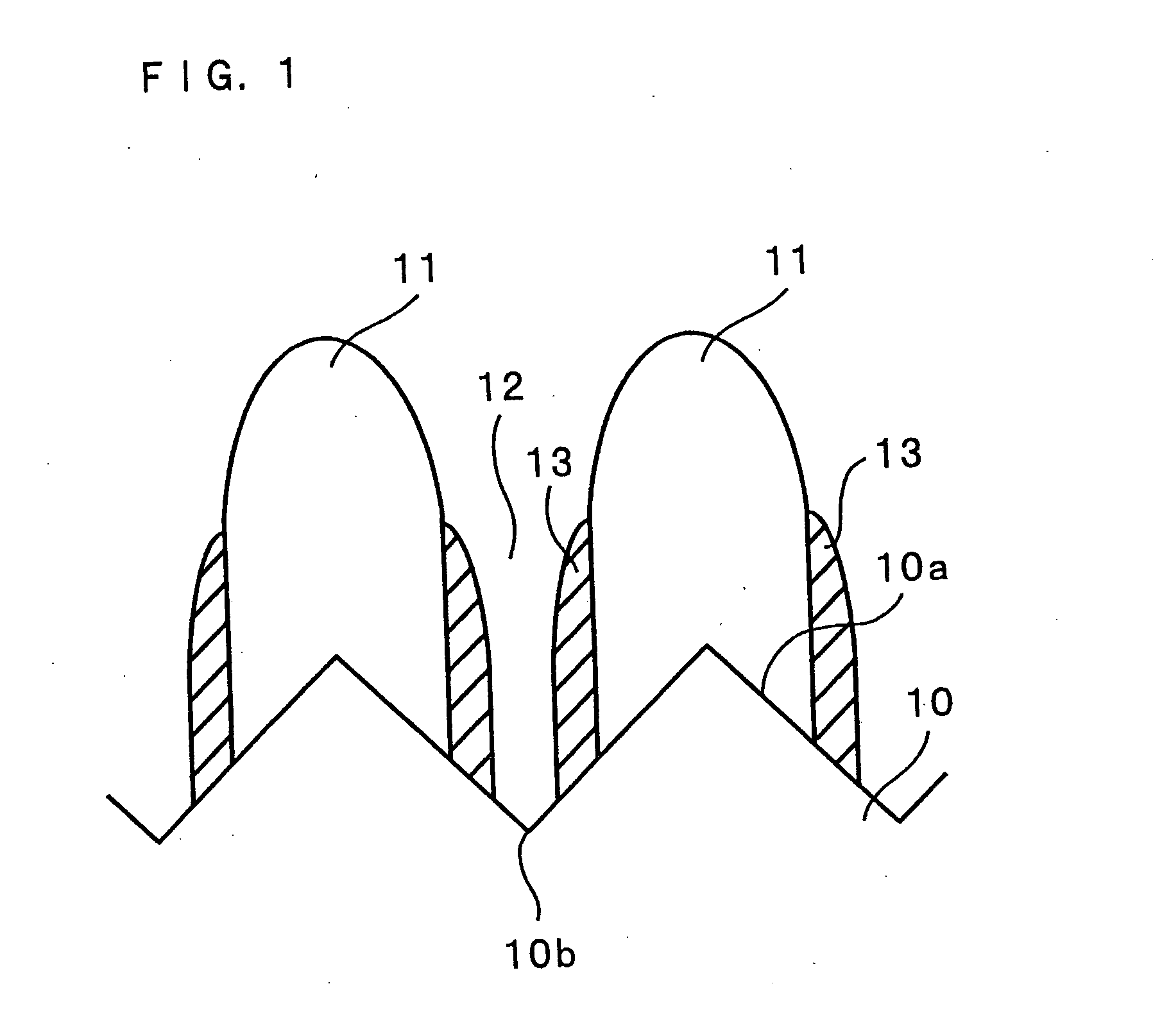
In order to enhance charge and discharge efficiency and to improve cycle characteristics by increasing a facing area between a positive electrode active material and a negative electrode active material, in a negative electrode for lithium secondary battery having a current collector and an active material layer carried on the current collector, the active material layer includes a plurality of columnar particles. The columnar particles include an element of silicon, and are tilted toward the normal direction of the current collector. Angle θ formed between the columnar particles and the normal direction of the current collector is preferably 10°≦θ<90°.
Owner:PANASONIC CORP
Method of making an ion-switching device without a separate lithiation step
InactiveUS20100007937A1Inhibition formationEfficient processingFinal product manufactureVacuum evaporation coatingChemical physicsElectrical conductor
In any manufacturing sequence for making a lithium ion-switching device, lithium has to be introduced at some stage into the device. An electrode inside the device is filled and depleted with lithium through an ion conductor at every use cycle of the device. Prior-art methods to introduce the lithium are: direct sputtering of lithium on the electrode, or electrochemically loading the electrode in an electrochemical cell, or indirectly loading the electrode after an ion conductor has been deposited on top of the electrode and still other methods. The inventive method disclosed makes such a separate lithiation step obsolete. The lithium is introduced at the same time as the ion-conductor is put on the electrode. This can be achieved by using an oxygen super-stoichiometric compound for the electrode.
Owner:SAGE ELECTROCHROMICS
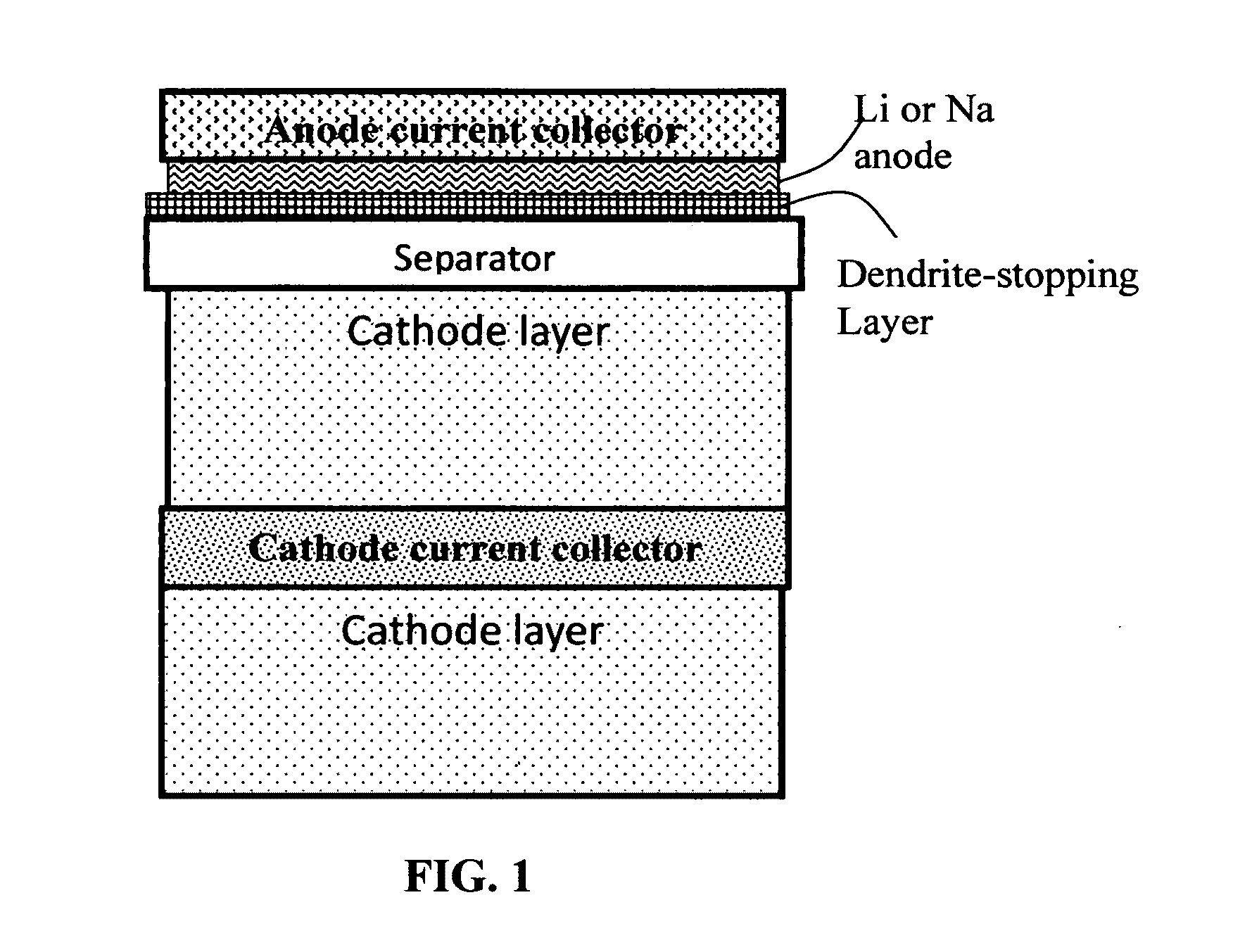
Alkali Metal Secondary Battery Containing a Carbon Matrix- or Carbon Matrix Composite-based Dendrite-Intercepting Layer
ActiveUS20160344035A1Increase energy densityReduce material costsFuel and secondary cellsPositive electrodesDendriteElectrolyte
A rechargeable alkali metal battery comprising: (a) an anode comprising an alkali metal layer and a dendrite penetration-resistant layer comprising an amorphous carbon or polymeric carbon matrix, an optional carbon or graphite reinforcement phase dispersed in this matrix, and a lithium- or sodium-containing species that are chemically bonded to the matrix and / or the optional carbon or graphite reinforcement to form an integral layer that prevents dendrite penetration, wherein the lithium- or sodium-containing species is selected from Li2CO3, Li2O, Li2C2O4, LiOH, LiX, ROCO2Li, HCOLi, ROLi, (ROCO2Li)2, (CH2OCO2Li)2, Li2S, LixSOy, Na2CO3, Na2O, Na2C2O4, NaOH, NaiX, ROCO2Na, HCONa, RONa, (ROCO2Na)2, (CH2OCO2Na)2, Na2S, NaxSOy, or a combination thereof, wherein X═F, Cl, I, or Br, R=a hydrocarbon group, x=0-1, y=1-4; (b) a cathode; and (c) a separator and electrolyte component; wherein the dendrite penetration-resistant layer is disposed between the alkali metal layer and the separator.
Owner:GLOBAL GRAPHENE GRP INC
Structured electrolyte for micro-battery
InactiveUS20060154141A1Fine surfaceSolid electrolytesFinal product manufactureOptoelectronicsElectrolyte
In order to increase the capacity of an “all-solid” type micro-battery, the layer of electrolyte is structured: transversing cavities are created in the flat layer, advantageously at the level of patches of collector material, then filled by anode or cathode material.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Methods and reagents for enhancing the cycling efficiency of lithium polymer batteries
InactiveUS6165644AImprove efficiencyElectrode rolling/calenderingElectrochemical processing of electrodesLithium metalSulfur electrode
Batteries including a lithium electrode and a sulfur counter electrode that demonstrate improved cycling efficiencies are described. In one embodiment, an electrochemical cell having a lithium electrode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The lithium electrode includes a surface coating that is effective to increase the cycling efficiency of said electrochemical cell. In a more particular embodiment, the lithium electrode is in an electrolyte solution, and, more particularly, an electrolyte solution including either elemental sulfur, a sulfide, or a polysulfide. In another embodiment, the coating is formed after the lithium electrode is contacted with the electrolyte. In a more particular embodiment, the coating is formed by a reaction between the lithium metal of the lithium electrode and a chemical species present in the electrolyte.
Owner:POLYPLUS BATTERY CO INC
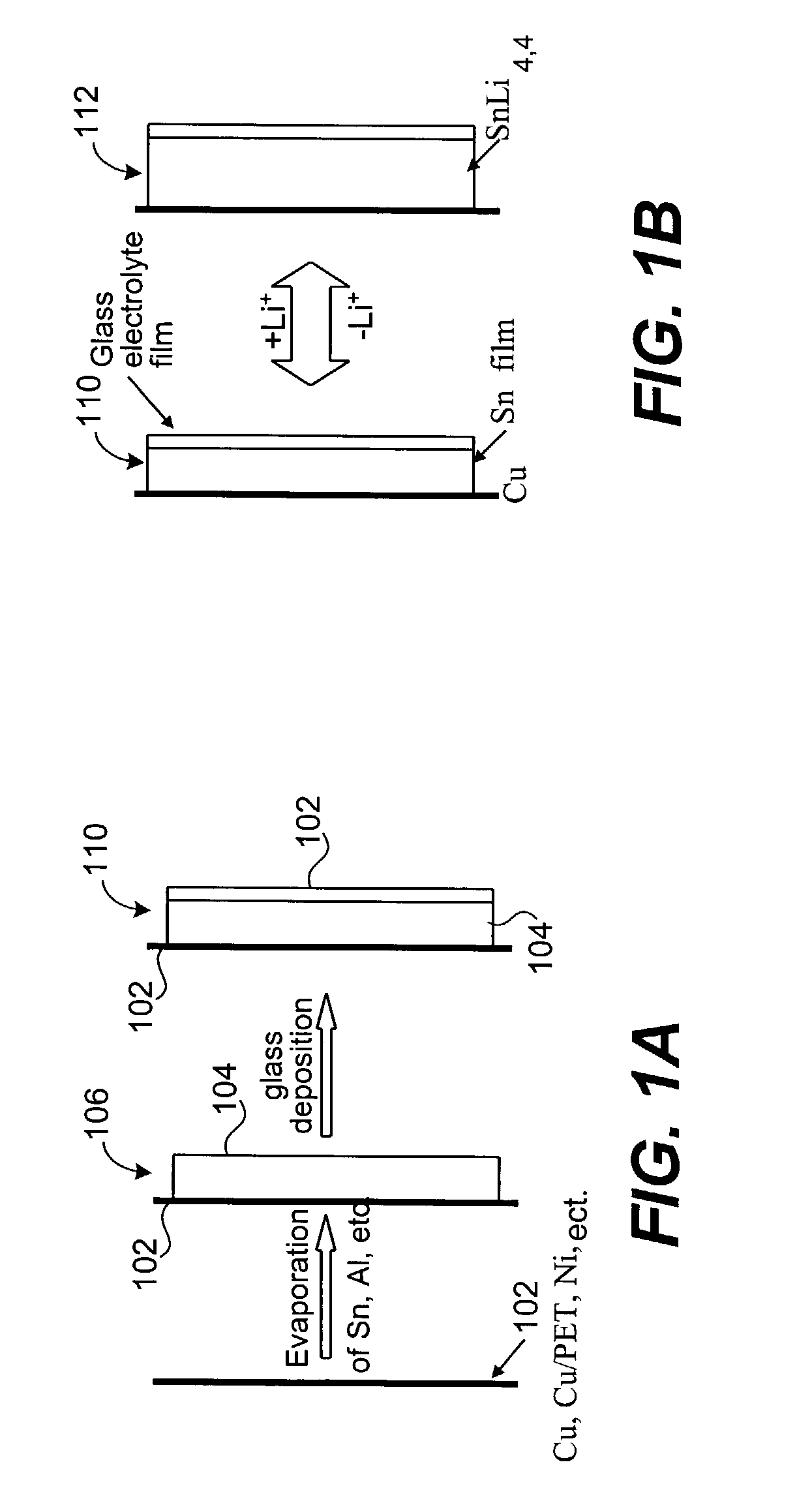
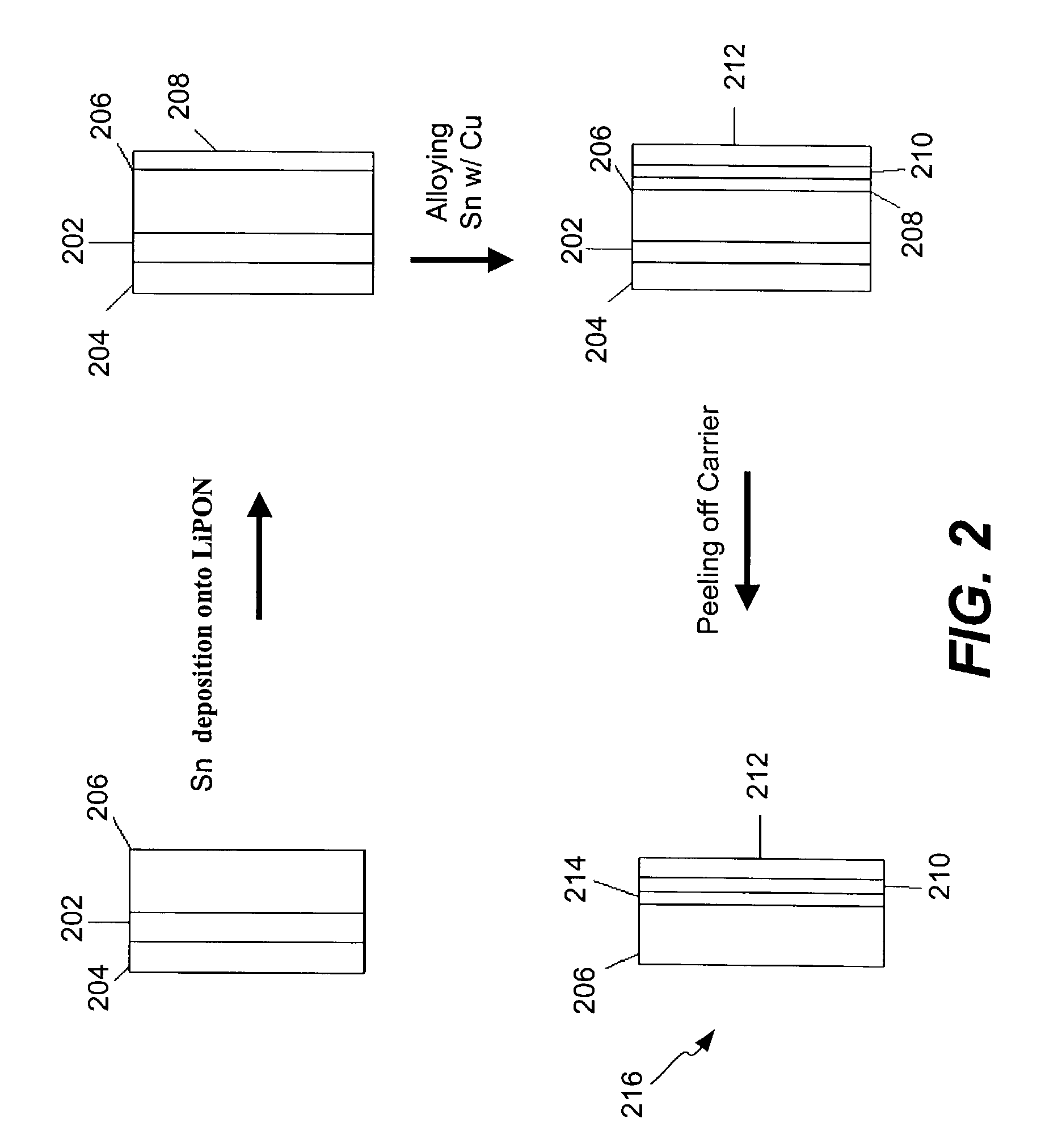
Coated lithium electrodes
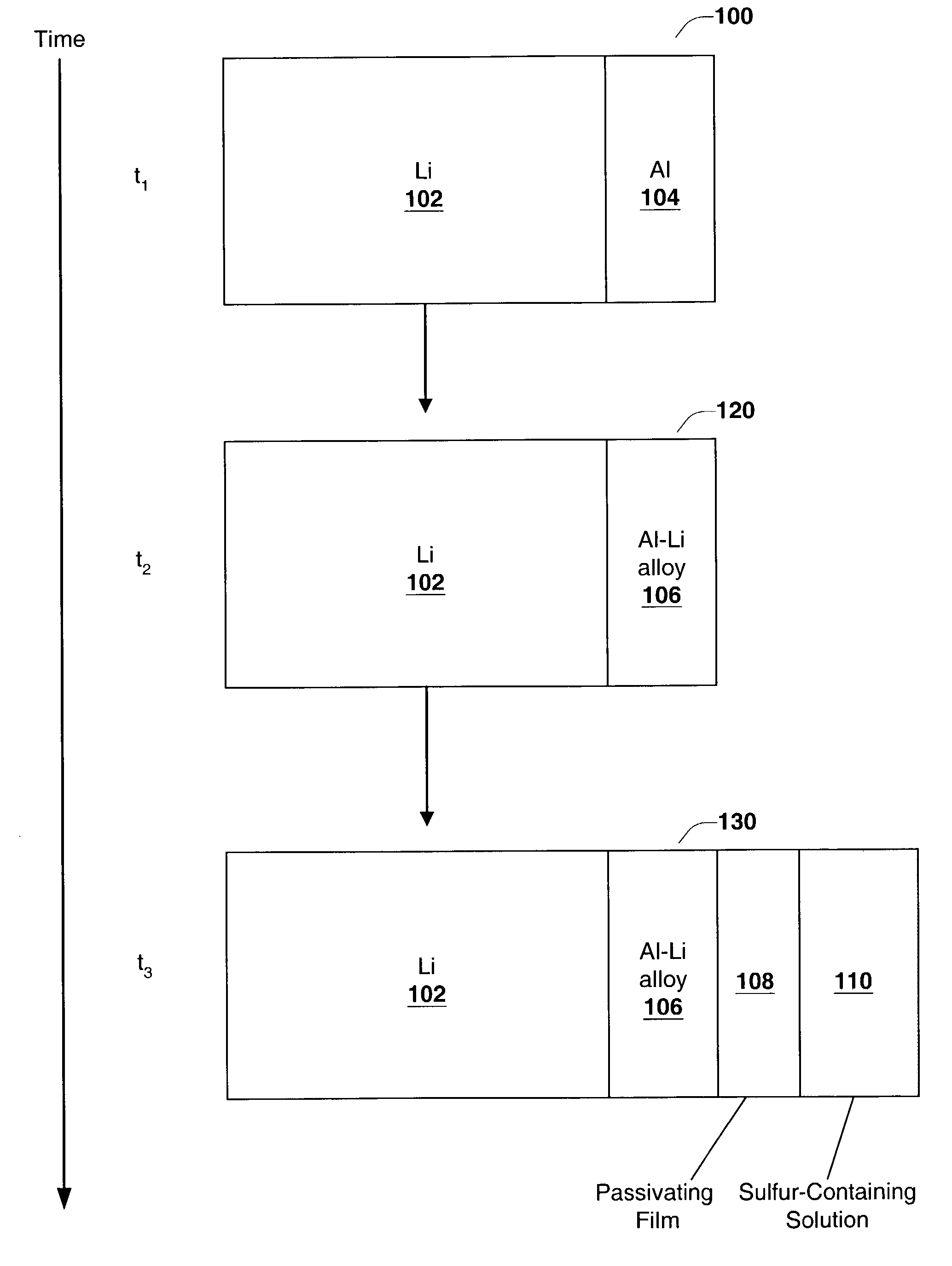
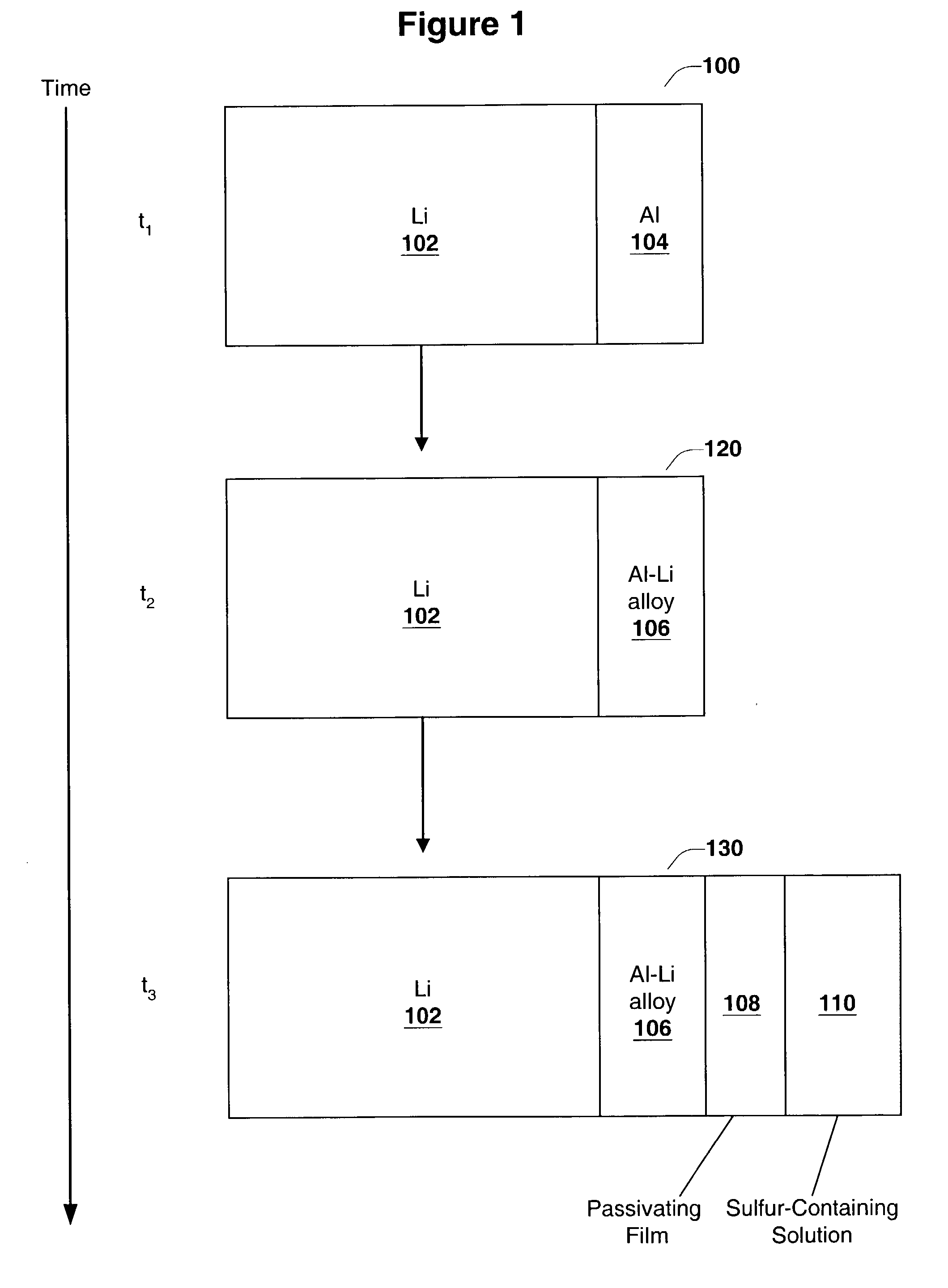
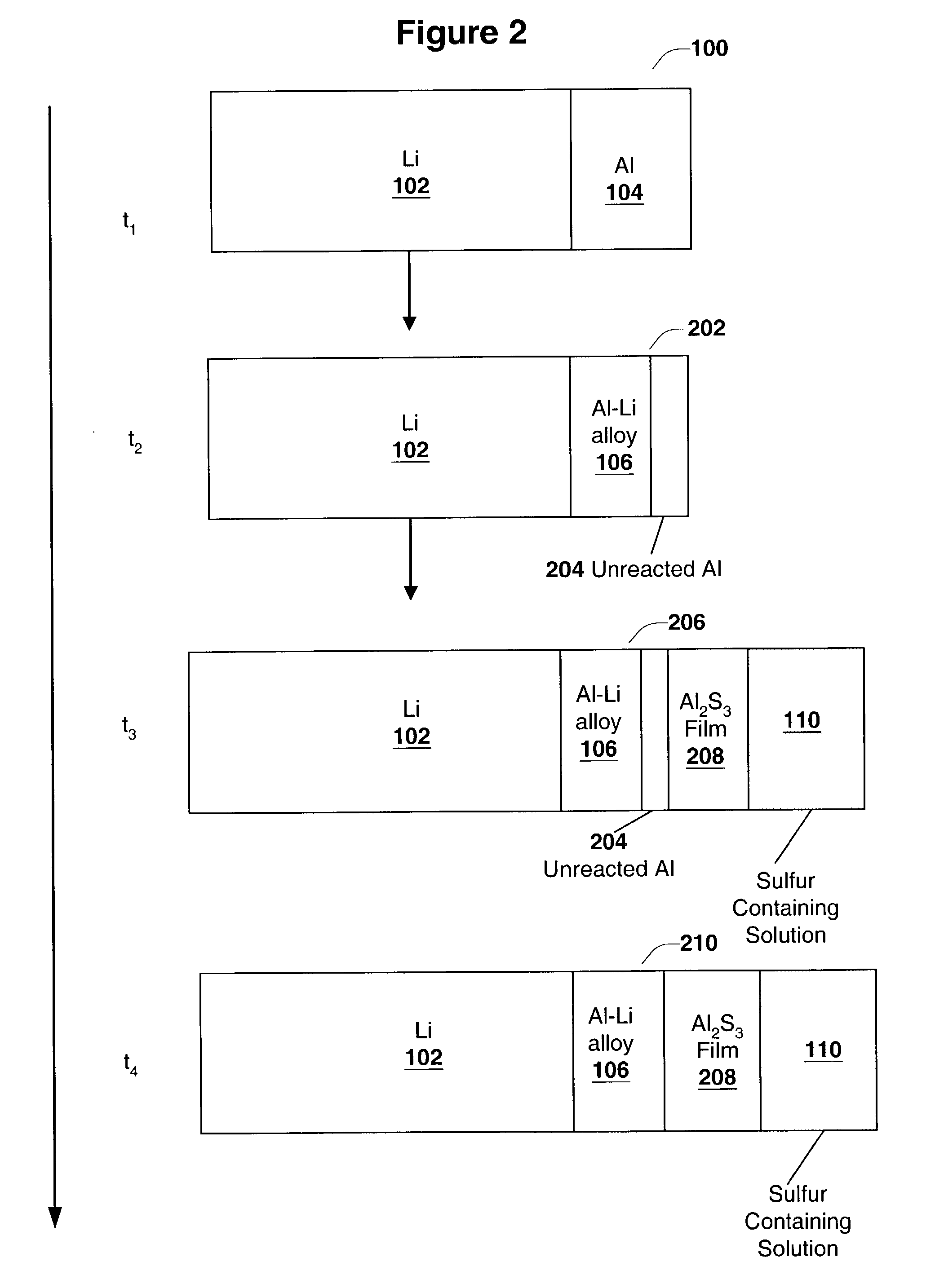
InactiveUS6955866B2Improve cycle lifeIncrease storageabilityCellsOrganic electrolyte cellsSulfur electrodePolysulfide
Batteries including a lithium anode stabilized with a metal-lithium alloy and battery cells comprising such anodes are provided. In one embodiment, an electrochemical cell having an anode and a sulfur electrode including at least one of elemental sulfur, lithium sulfide, and a lithium polysulfide is provided. The anode includes a lithium core and a ternary alloy layer over the lithium core where the ternary alloy comprises lithium and two other metals. The ternary alloy layer is effective to increase cycle life and storageability of the electrochemical cell. In a more particular embodiment, the ternary alloy layer is comprised of lithium, copper and tin.
Owner:POLYPLUS BATTERY CO INC
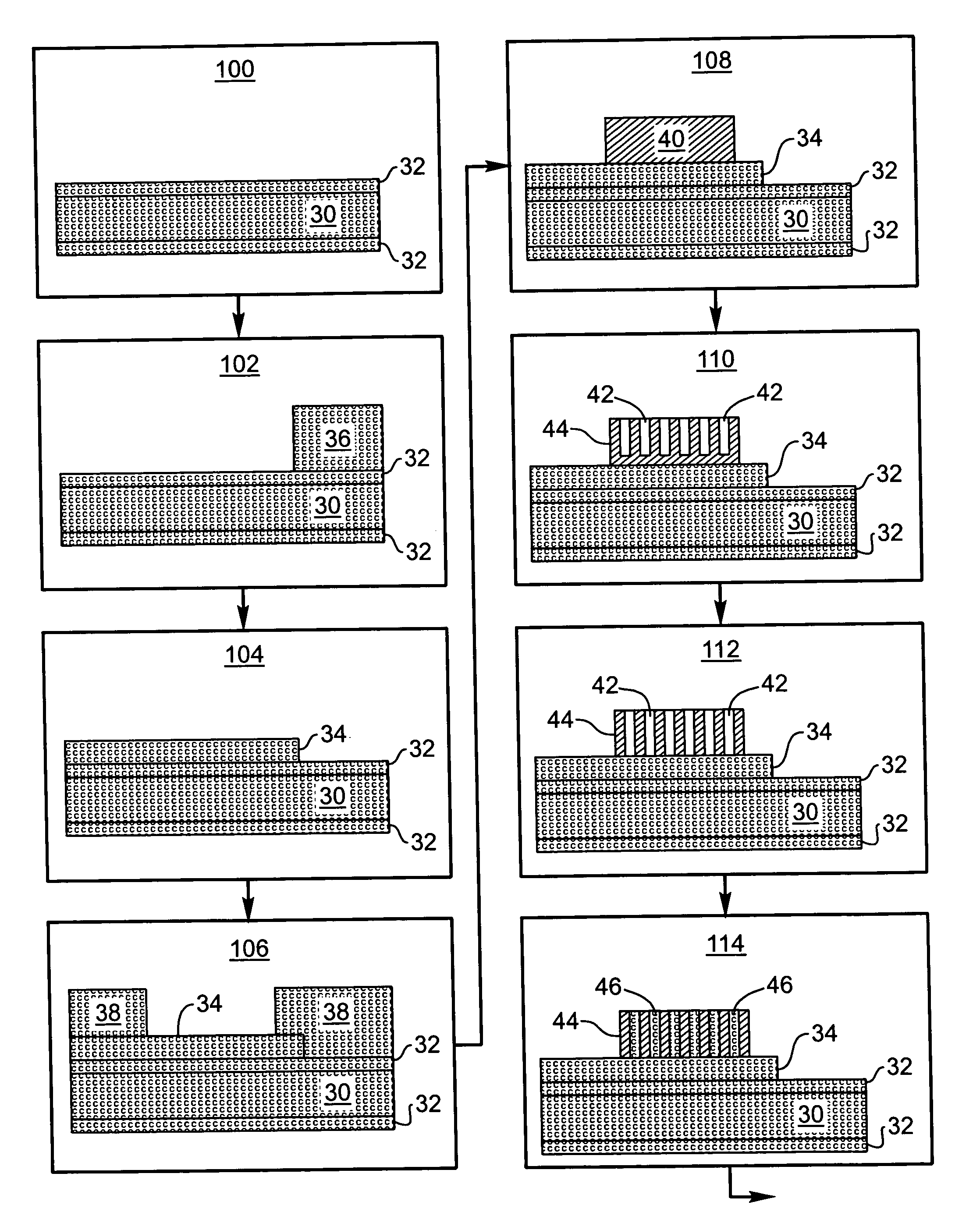
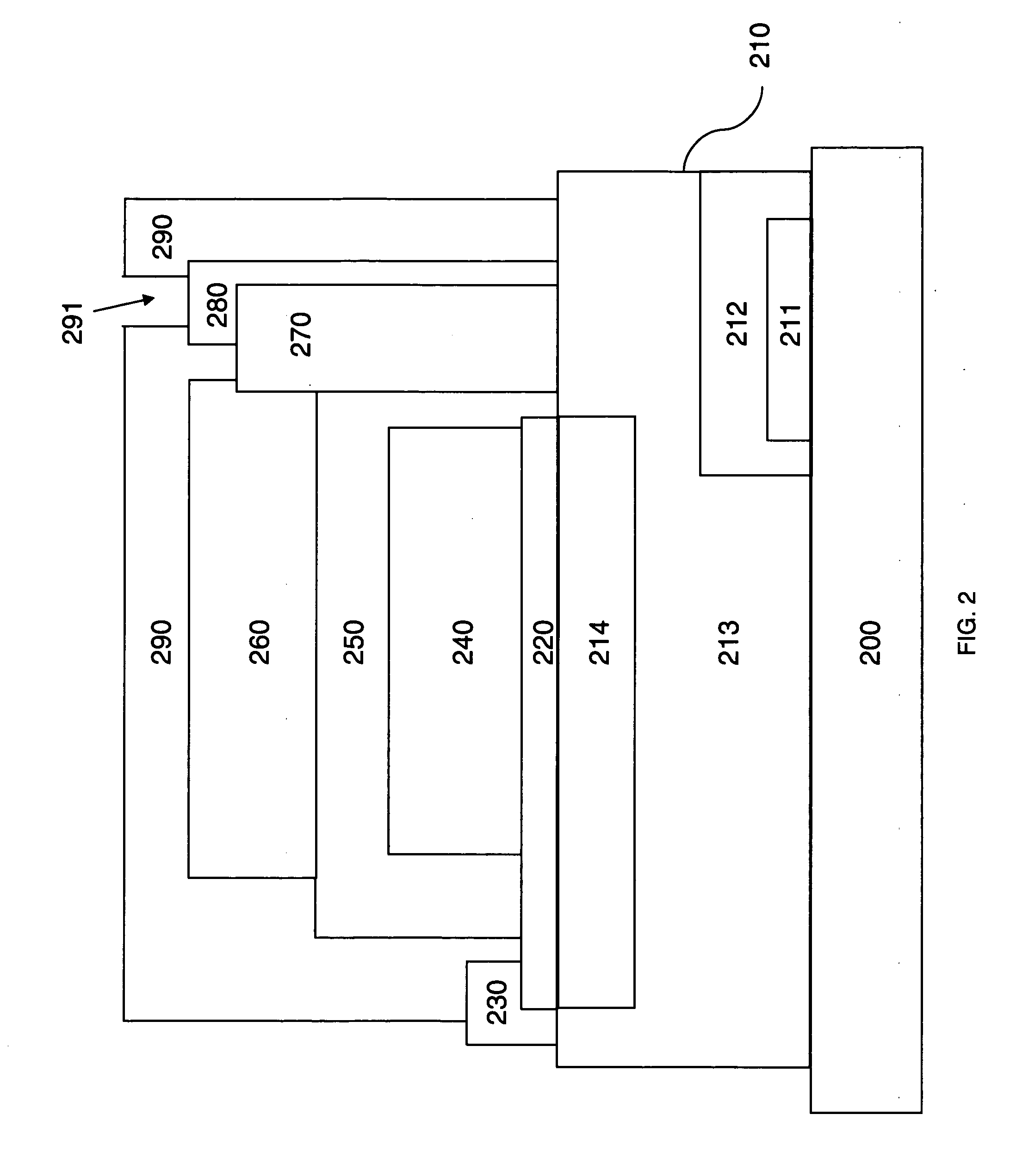
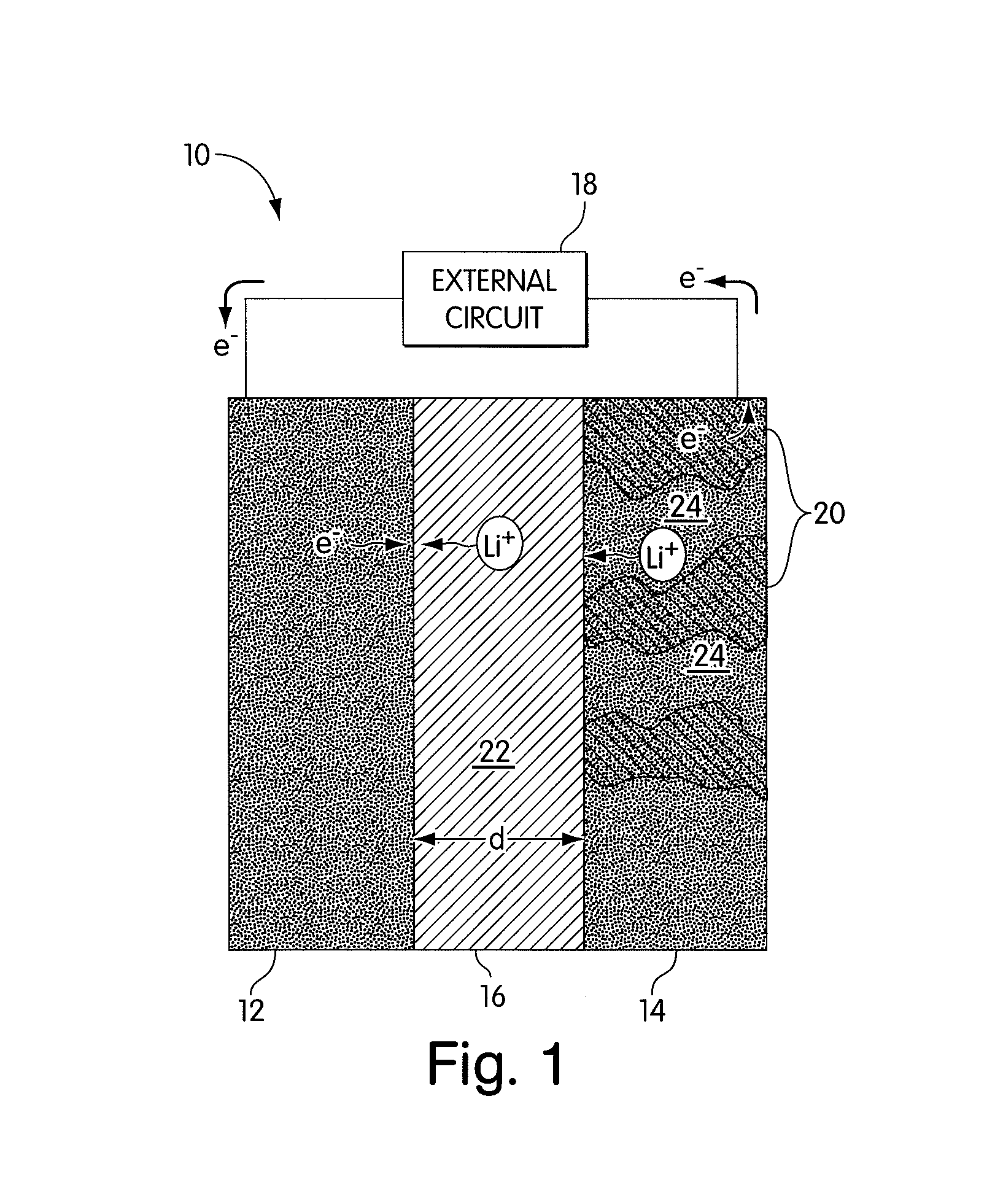
Electrode structure and method for making the same
Electrode structures, and more specifically, electrode structures for use in electrochemical cells, are provided. The electrode structures described herein may include one or more protective layers. In one set of embodiments, a protective layer may be formed by exposing a lithium metal surface to a plasma comprising ions of a gas to form a ceramic layer on top of the lithium metal. The ceramic layer may be highly conductive to lithium ions and may protect the underlying lithium metal surface from reaction with components in the electrolyte. In some cases, the ions may be nitrogen ions and a lithium nitride layer may be formed on the lithium metal surface. In other embodiments, the protective layer may be formed by converting lithium to lithium nitride at high pressures. Other methods for forming protective layers are also provided.
Owner:SION POWER CORP
Method for covering particles, especially a battery electrode material particles, and particles obtained with such method and a battery comprising such particle
A method for covering particles having a diameter of maximally 60 μm by means of atomic layer deposition, whereby said method comprises the step of fluidizing said particles in a fluidized bed reactor using a first reactant gas comprising a first reactant for substantially completely covering said particles with a monolayer of said first reactant.
Owner:PNEUMATICOAT TECH LLC
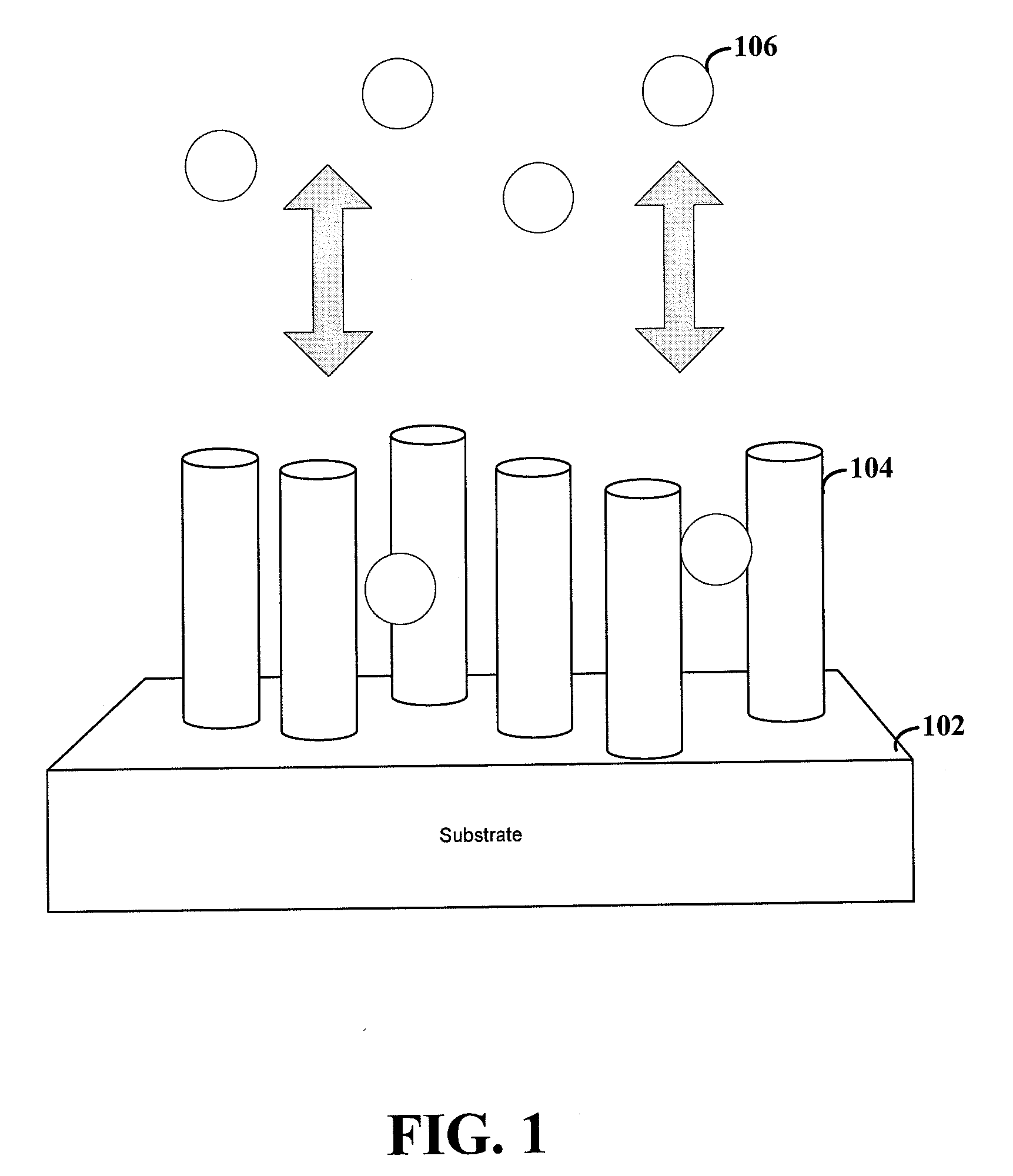
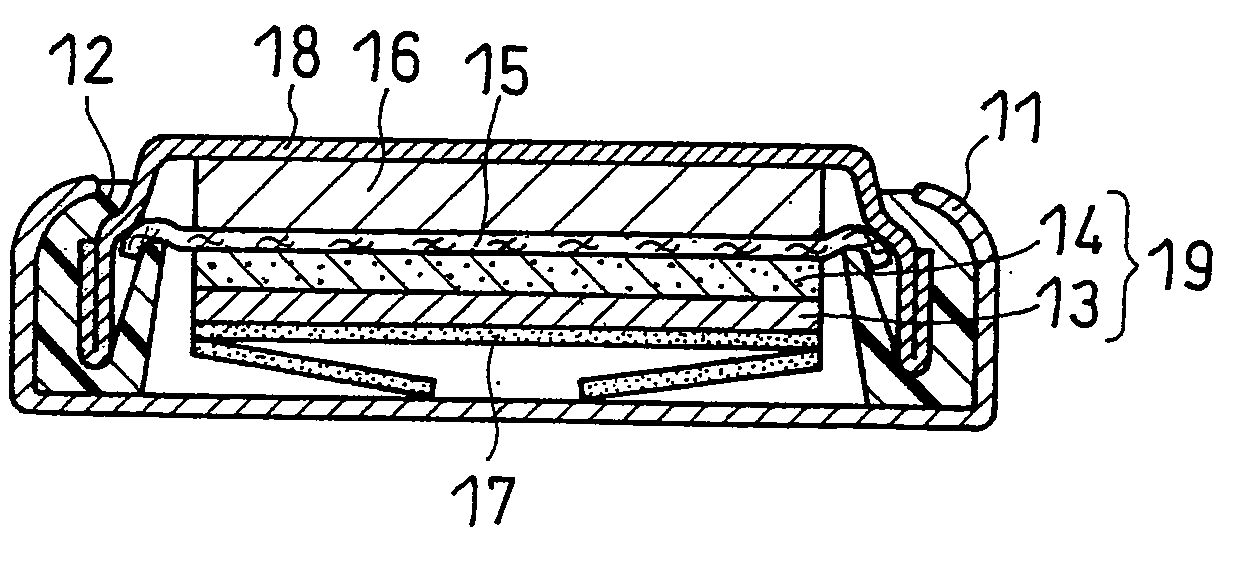
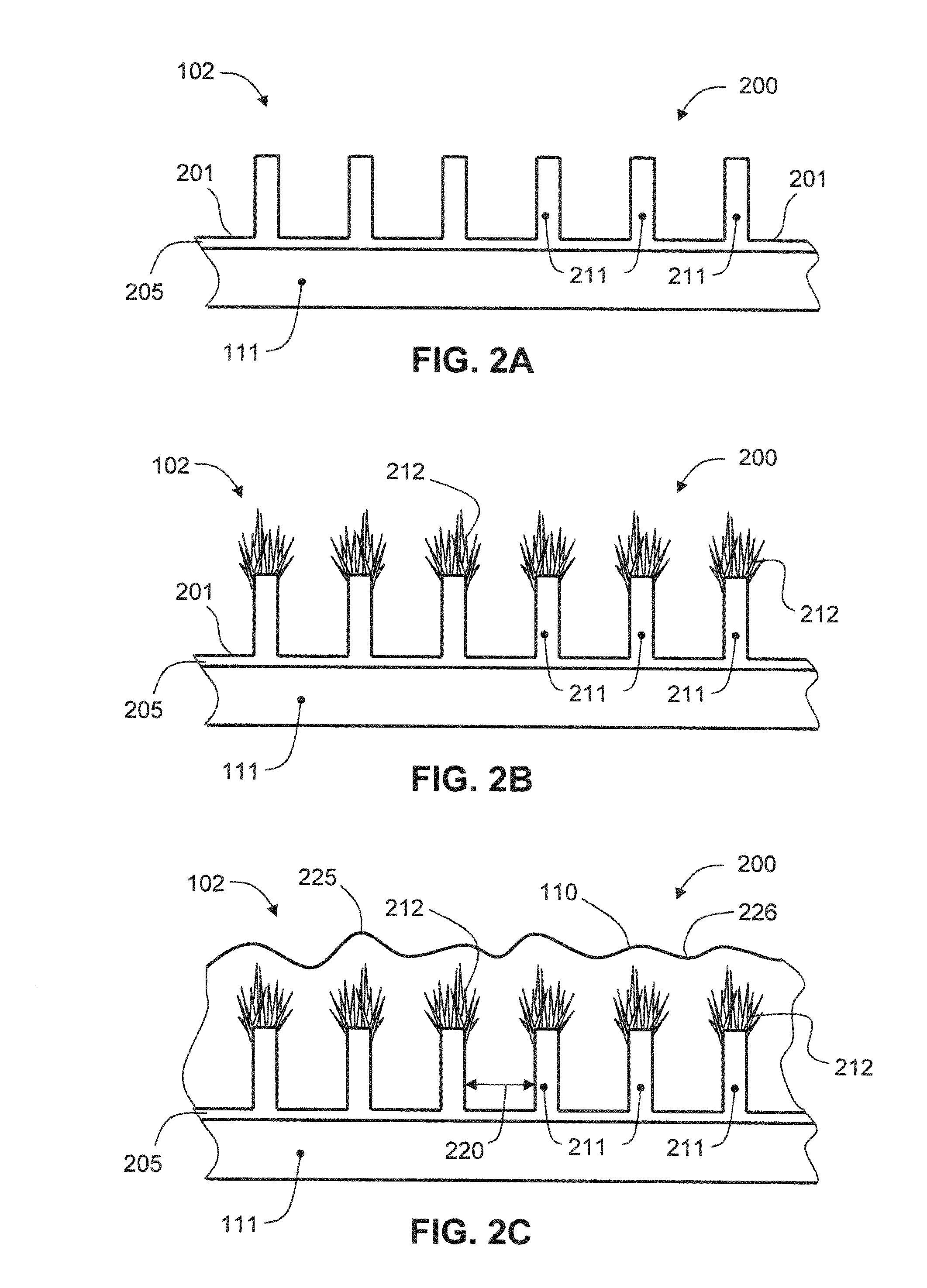
Nanostructured Electrode for a Microbattery
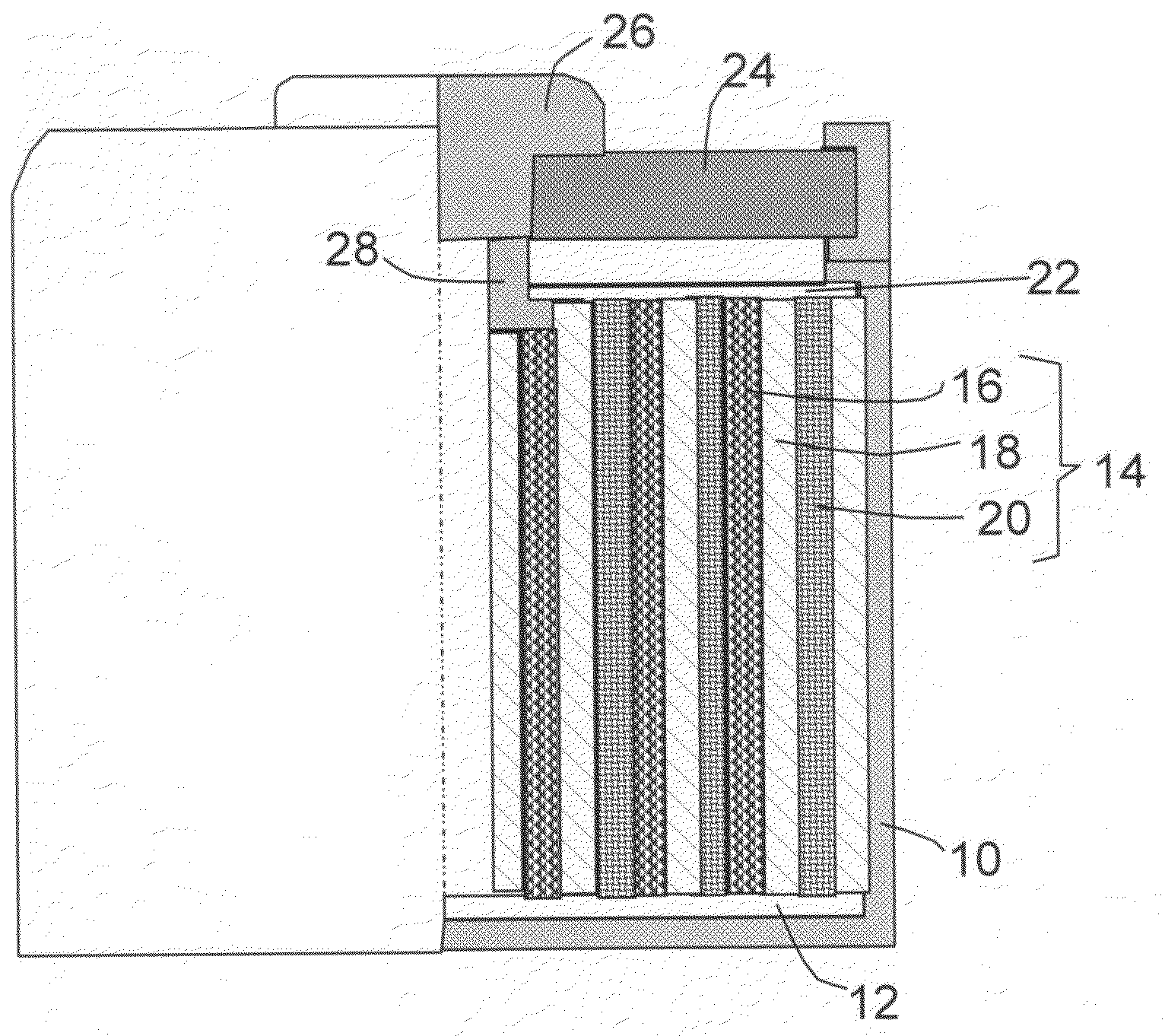
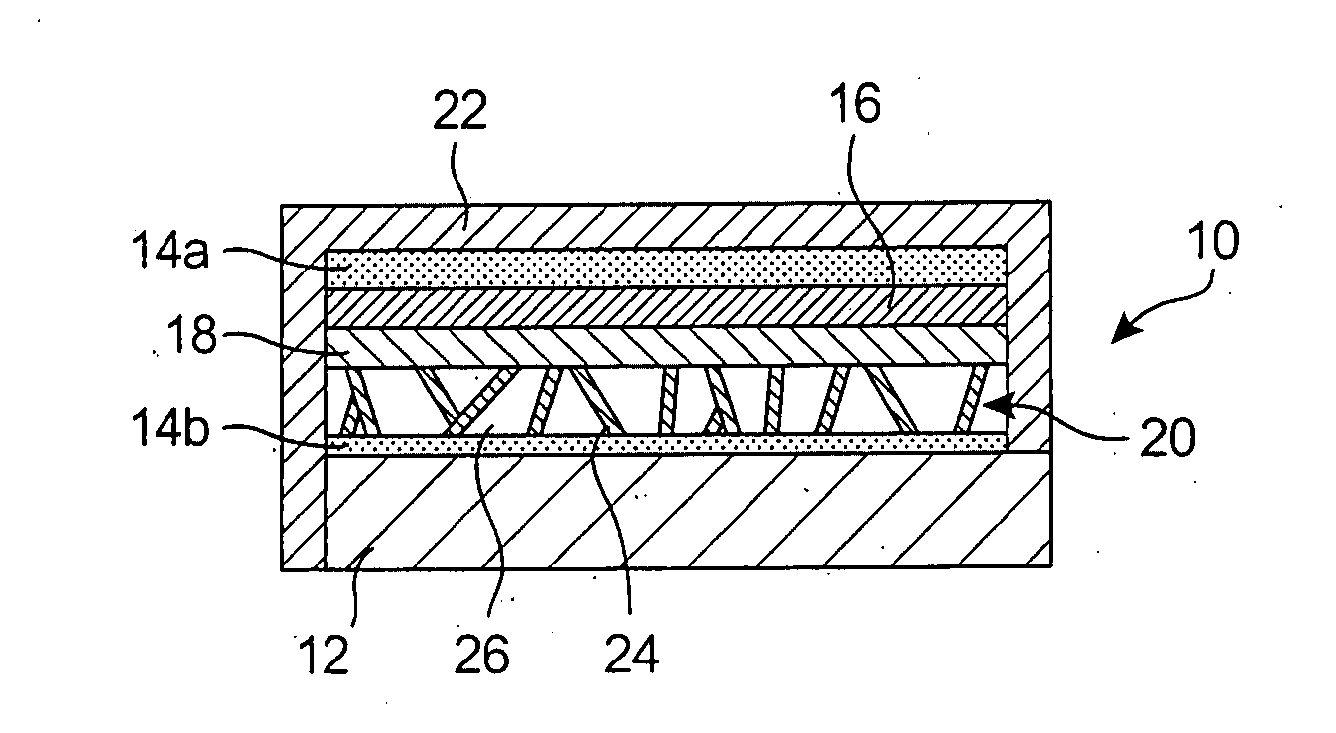
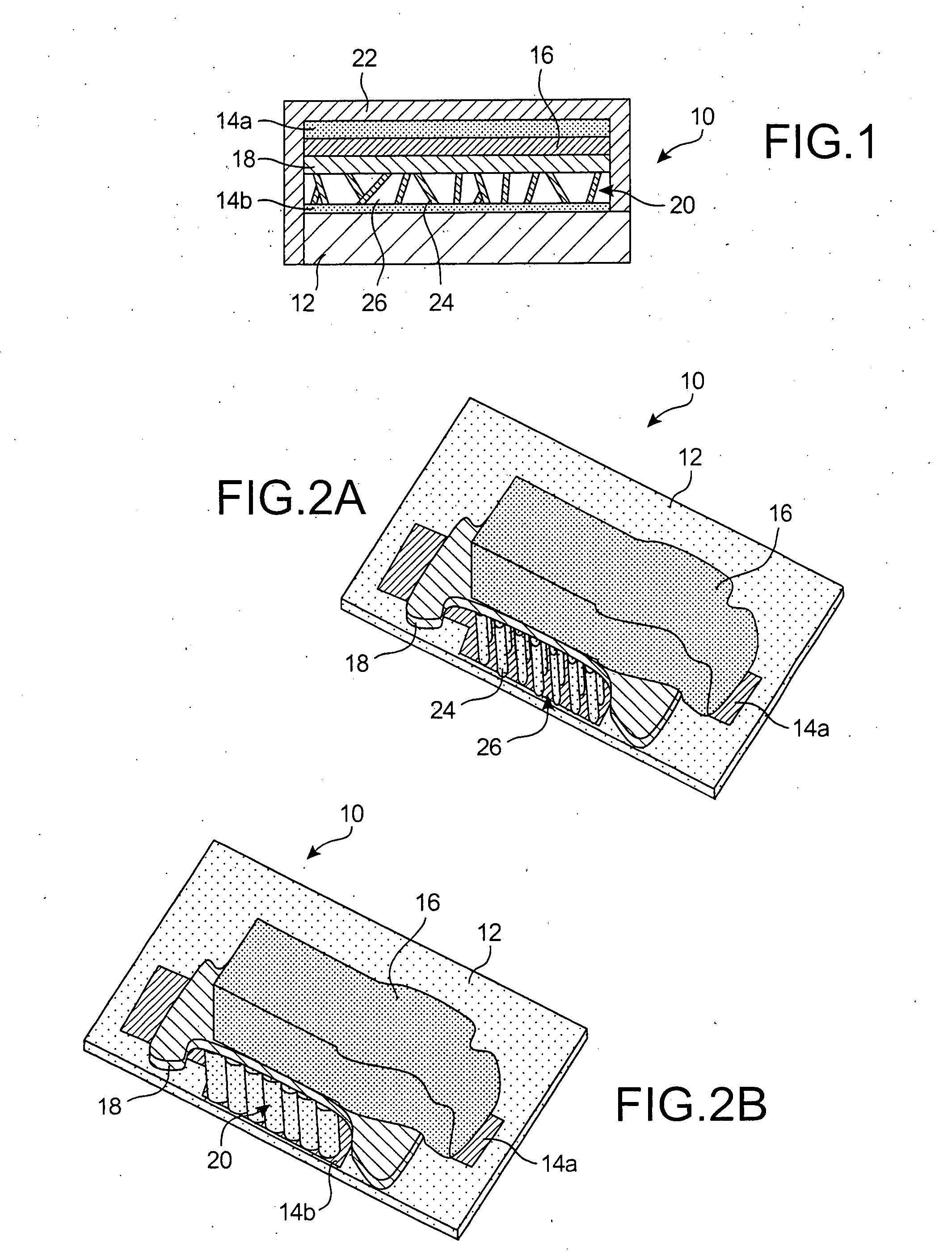
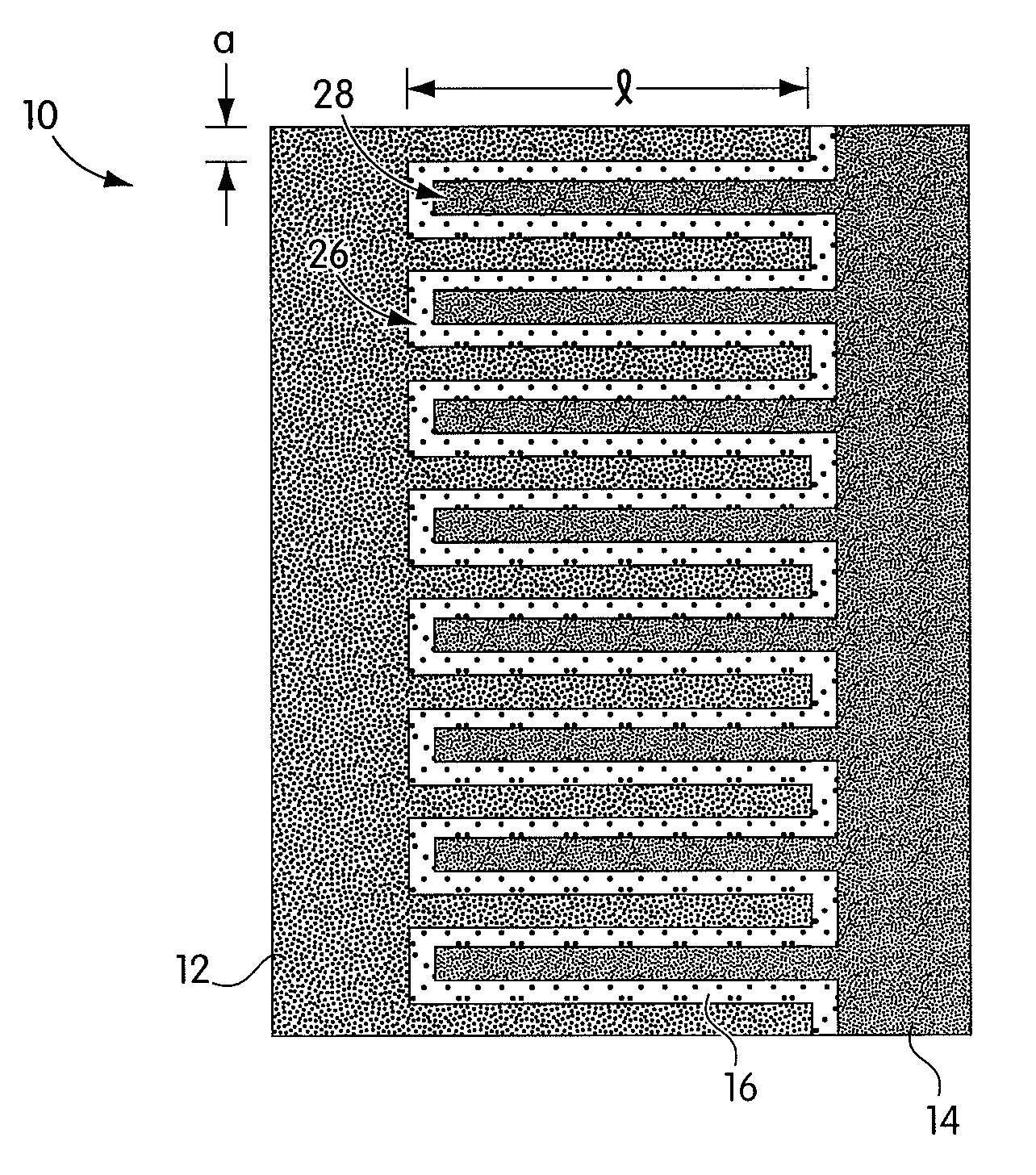
InactiveUS20080044732A1Overcome problemsSuppress stressMaterial nanotechnologySolid electrolytesLithiumNanowire
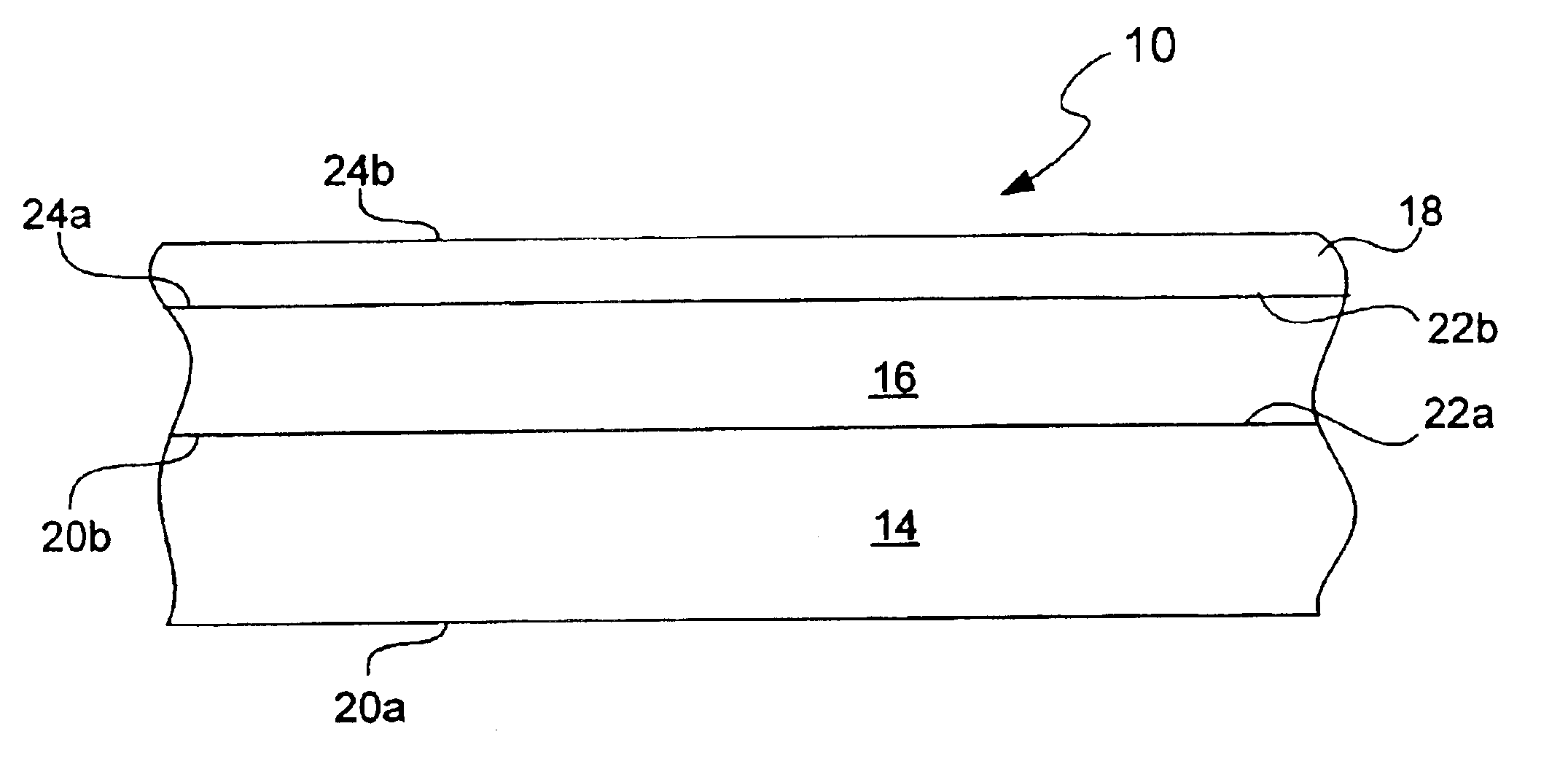
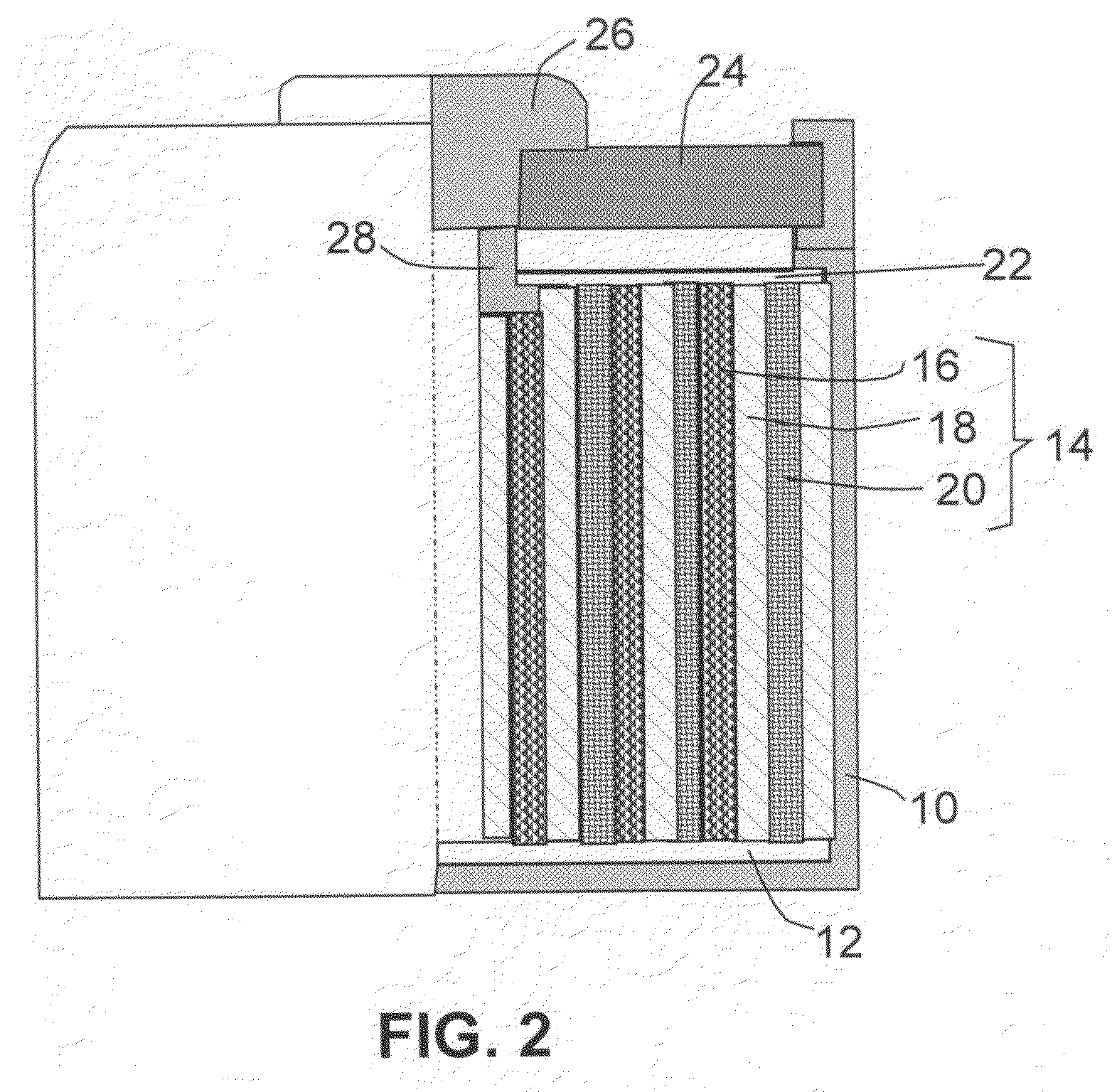
A new anode configuration (20) is proposed for a lithium microbattery (10). The anode (20) preferably consists of nanotubes or of nanowires (24) such that the empty space (26) left between the different components (24) provides compensation for the inherent swelling upon discharging the microbattery (10). With the absence of stresses on the electrolyte (18), the lifetime of the battery (10) may be increased.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Reticulated and controlled porosity battery structures
InactiveUS7553584B2Boost energyHigh porosityElectrode thermal treatmentElectrolytic capacitorsEngineeringTortuous retinal vessels
The effective ionic conductivity in a composite structure is believed to decrease rapidly with volume fraction. A system, such as a bipolar device or energy storage device, has structures or components in which the diffusion length or path that electrodes or ions must traverse is minimized and the interfacial area exposed to the ions or electrons is maximized. The device includes components that can be reticulated or has a reticulated interface so that an interface area can be increased. The increased interfacial perimeter increases the available sites for reaction of ionic species. Many different reticulation patterns can be used. The aspect ratio of the reticulated features can be varied. Such bipolar devices can be fabricated by a variety of methods or procedures. A bipolar device having structures of reticulated interface can be tailored for the purposes of controlling and optimizing charge and discharge kinetics. A bipolar device having graded porosity structures can have improved transport properties because the diffusion controlling reaction kinetics can be modified. Graded porosity electrodes can be linearly or nonlinearly graded. A bipolar device having perforated structures also provides improved transport properties by removing tortuosity and reducing diffusion distance.
Owner:MASSACHUSETTS INST OF TECH
Non-aqueous electrolyte secondary battery negative electrode material, making method, lithium ion secondary battery, and electrochemical capacitor
InactiveUS20090202911A1Improve cycle performanceLarge capacityHybrid capacitor electrodesLiquid electrolytic capacitorsGraphiteCapacitor
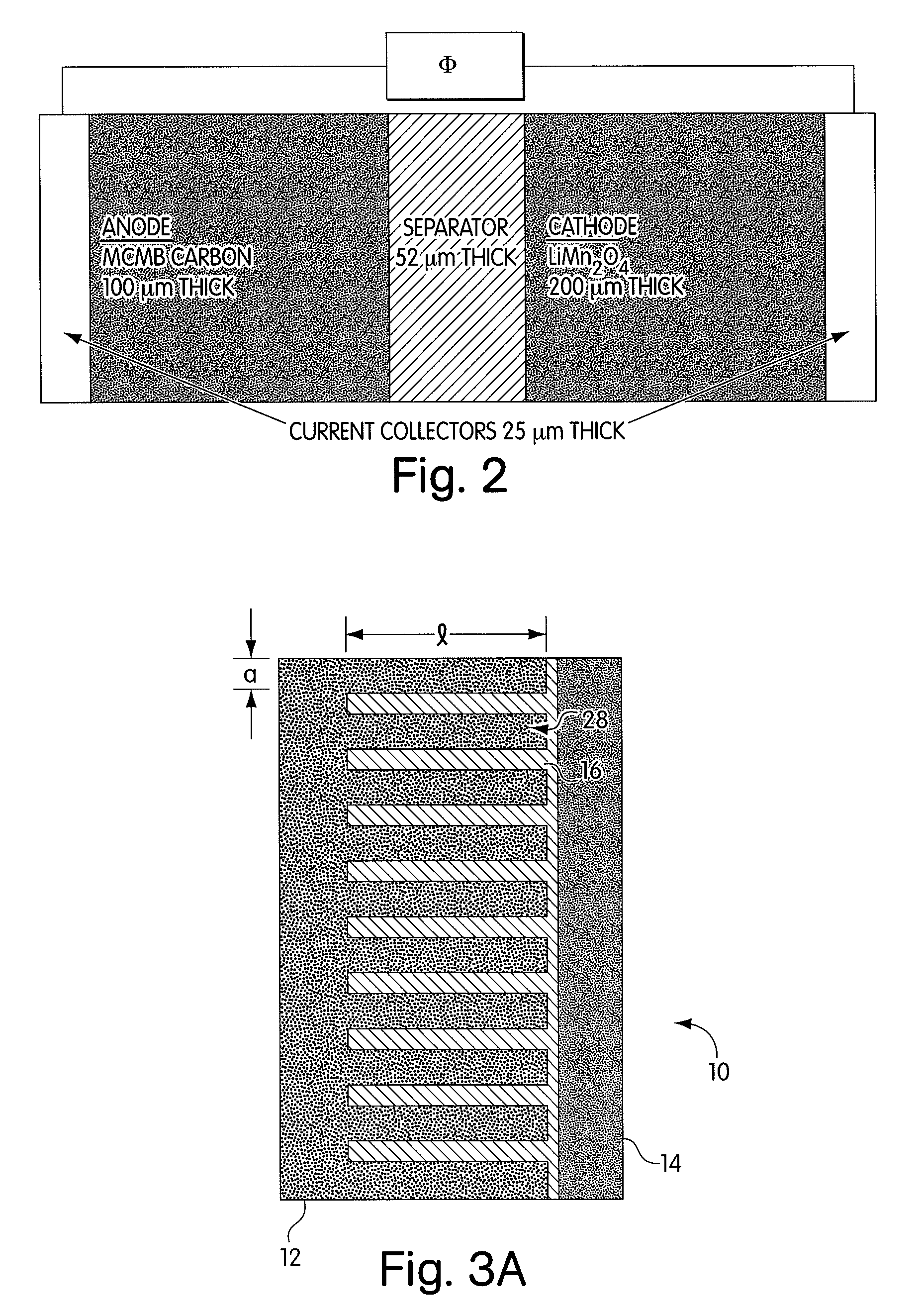
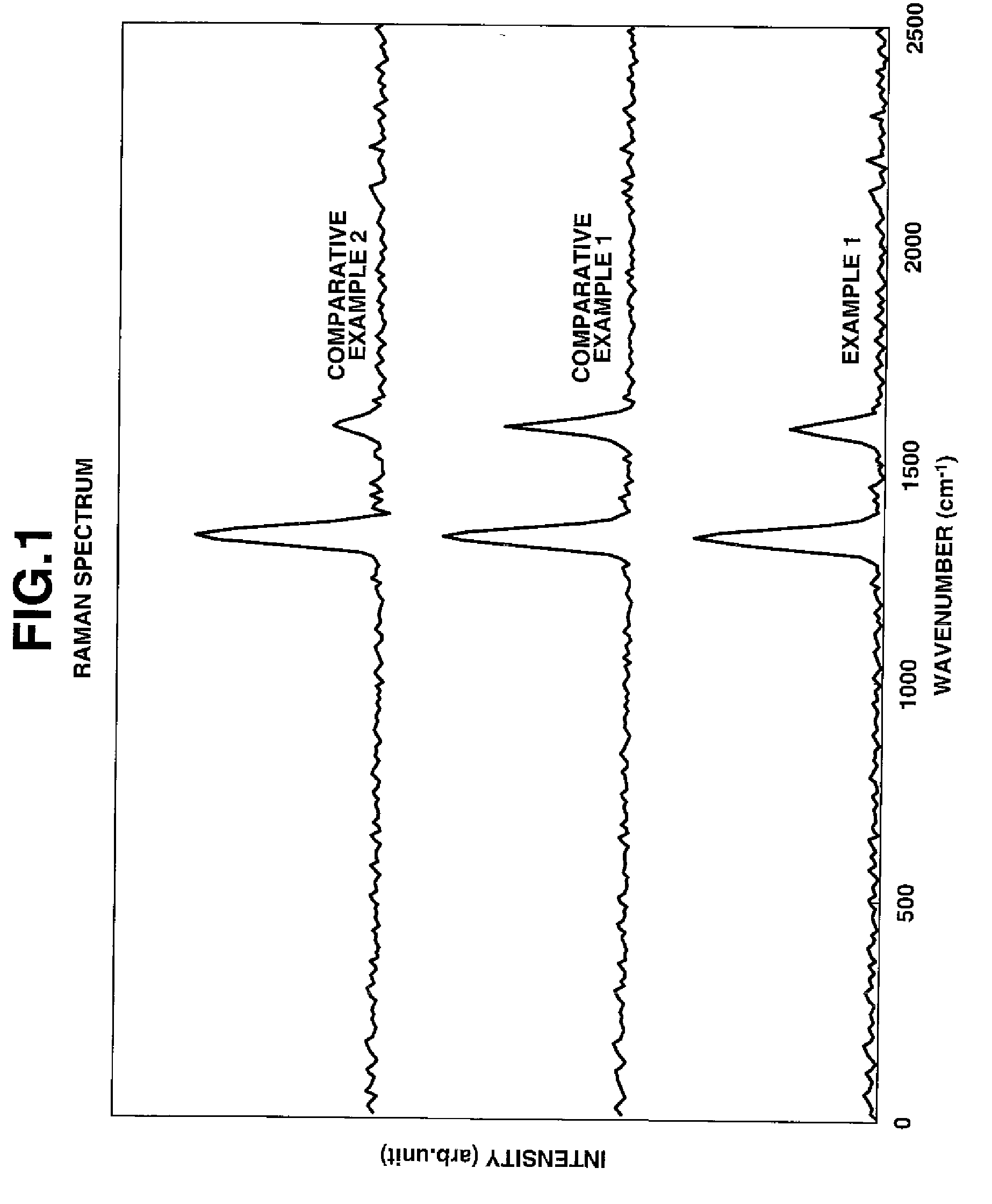
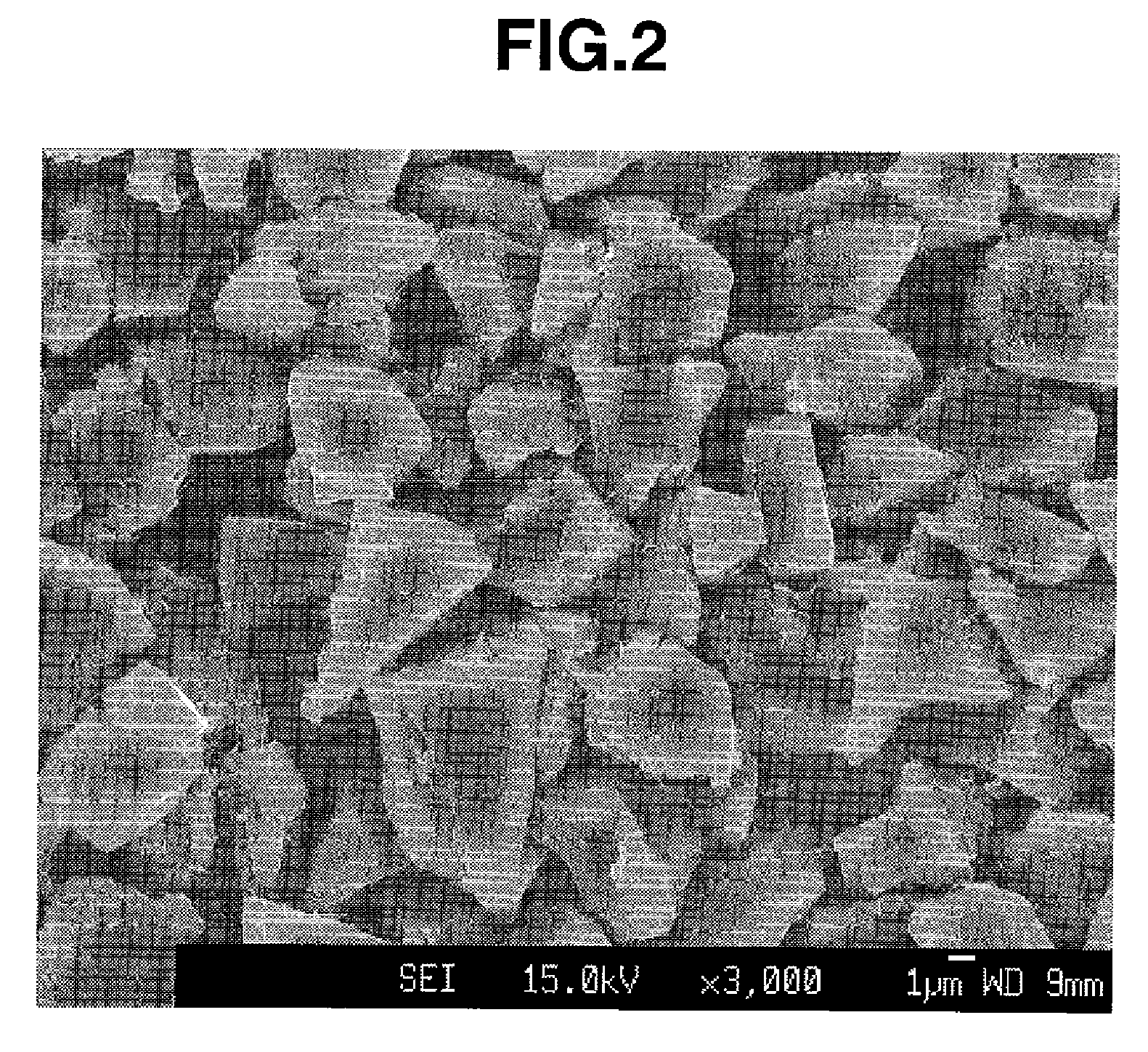
A negative electrode material comprises a conductive powder of particles of a lithium ion-occluding and releasing material coated on their surface with a graphite coating. The graphite coating, on Raman spectroscopy analysis, develops broad peaks having an intensity I1330 and I1580 at 1330 cm−1 and 1580 cm−1 Raman shift, an intensity ratio I1330 / I1580 being 1.5<I1330 / I1580<3.0. Using the negative electrode material, a lithium ion secondary battery having a high capacity and improved cycle performance can be manufactured.
Owner:SHIN ETSU CHEM IND CO LTD
Lithium anodes for electrochemical cells
InactiveUS20080014501A1Light weightFinal product manufactureElectrode carriers/collectorsLithium metalReactive gas
Provided is an anode for use in electrochemical cells, wherein the anode active layer has a first layer comprising lithium metal and a multi-layer structure comprising single ion conducting layers and polymer layers in contact with the first layer comprising lithium metal or in contact with an intermediate protective layer, such as a temporary protective metal layer, on the surface of the lithium-containing first layer. Another aspect of the invention provides an anode active layer formed by the in-situ deposition of lithium vapor and a reactive gas. The anodes of the current invention are particularly useful in electrochemical cells comprising sulfur-containing cathode active materials, such as elemental sulfur.
Owner:SION POWER CORP
Lithium secondary battery
InactiveUS20050008939A1Improve featuresElectrochemical processing of electrodesFinal product manufactureLithiumPhosphate
A lithium secondary battery comprising a positive electrode, a negative electrode, and a nonaqueous electrolyte, wherein the positive electrode or the negative electrode is an electrode obtained by depositing a thin film of active material capable of lithium storage and release on a current collector, the thin film is divided into columns by gaps formed therein in a manner to extend in its thickness direction and the columnar portions are adhered at their bottoms to the current collector, and the nonaqueous electrolyte contains at least one selected from phosphate ester, phosphite ester, borate ester and carboxylic ester having a fluoroalkyl group.
Owner:SANYO ELECTRIC CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com