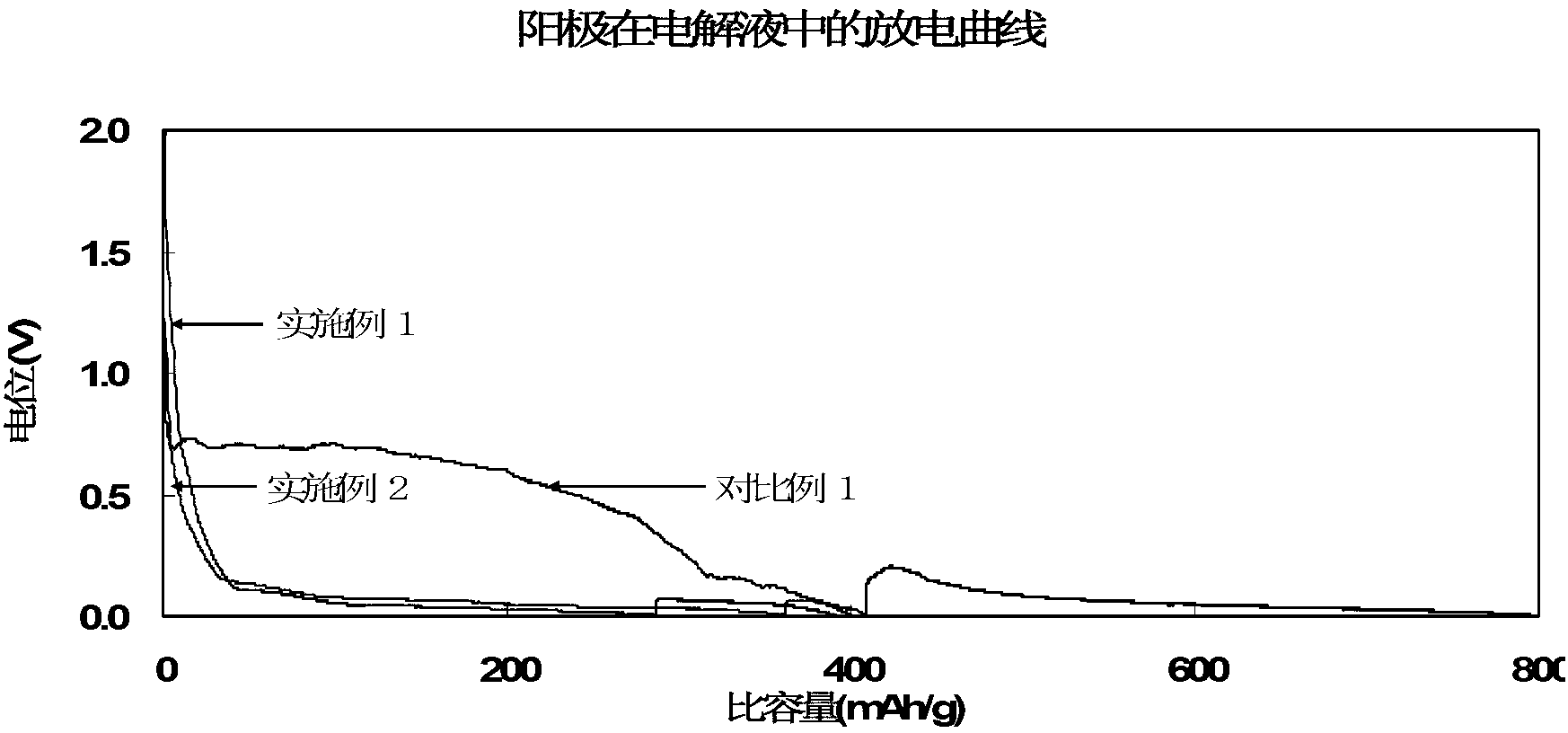
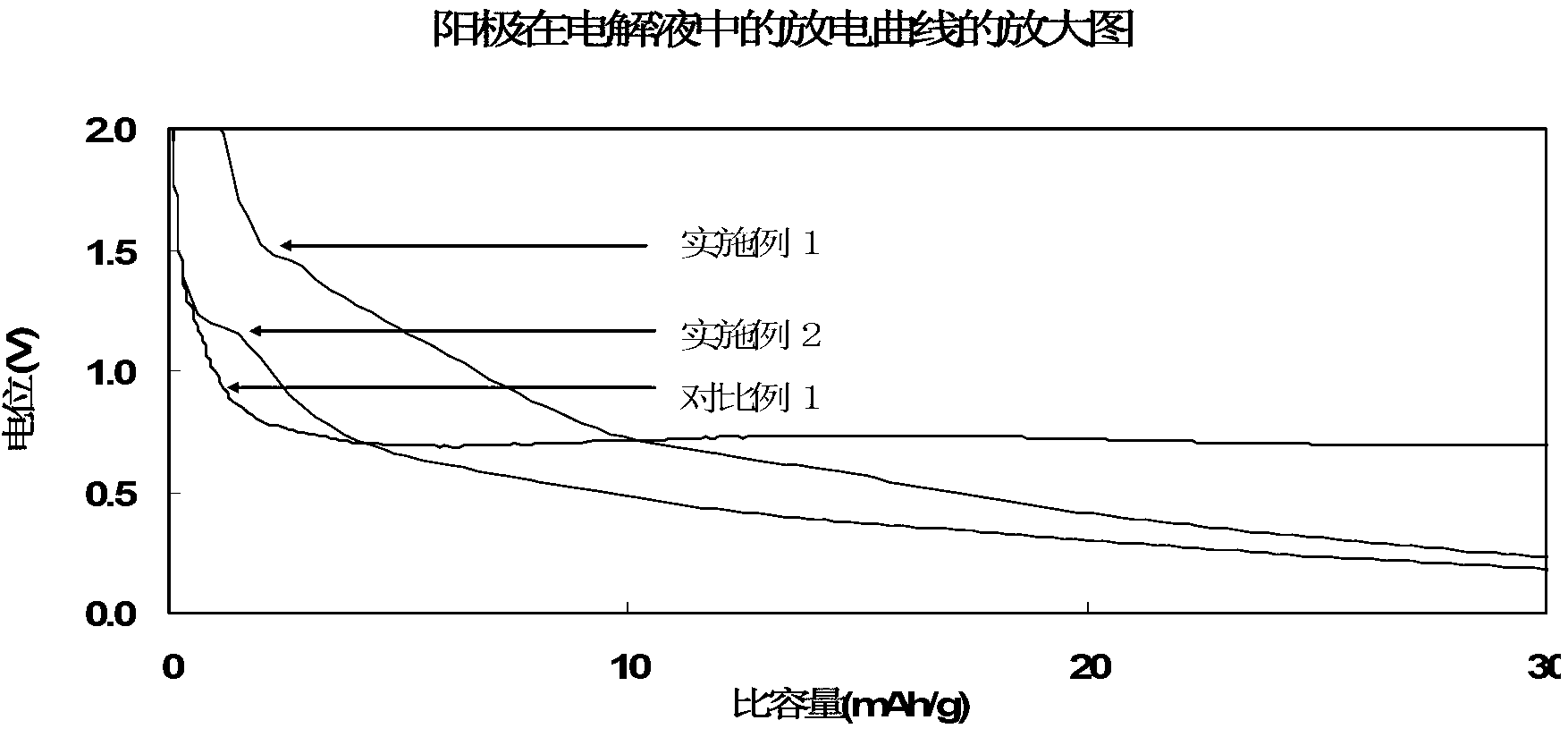
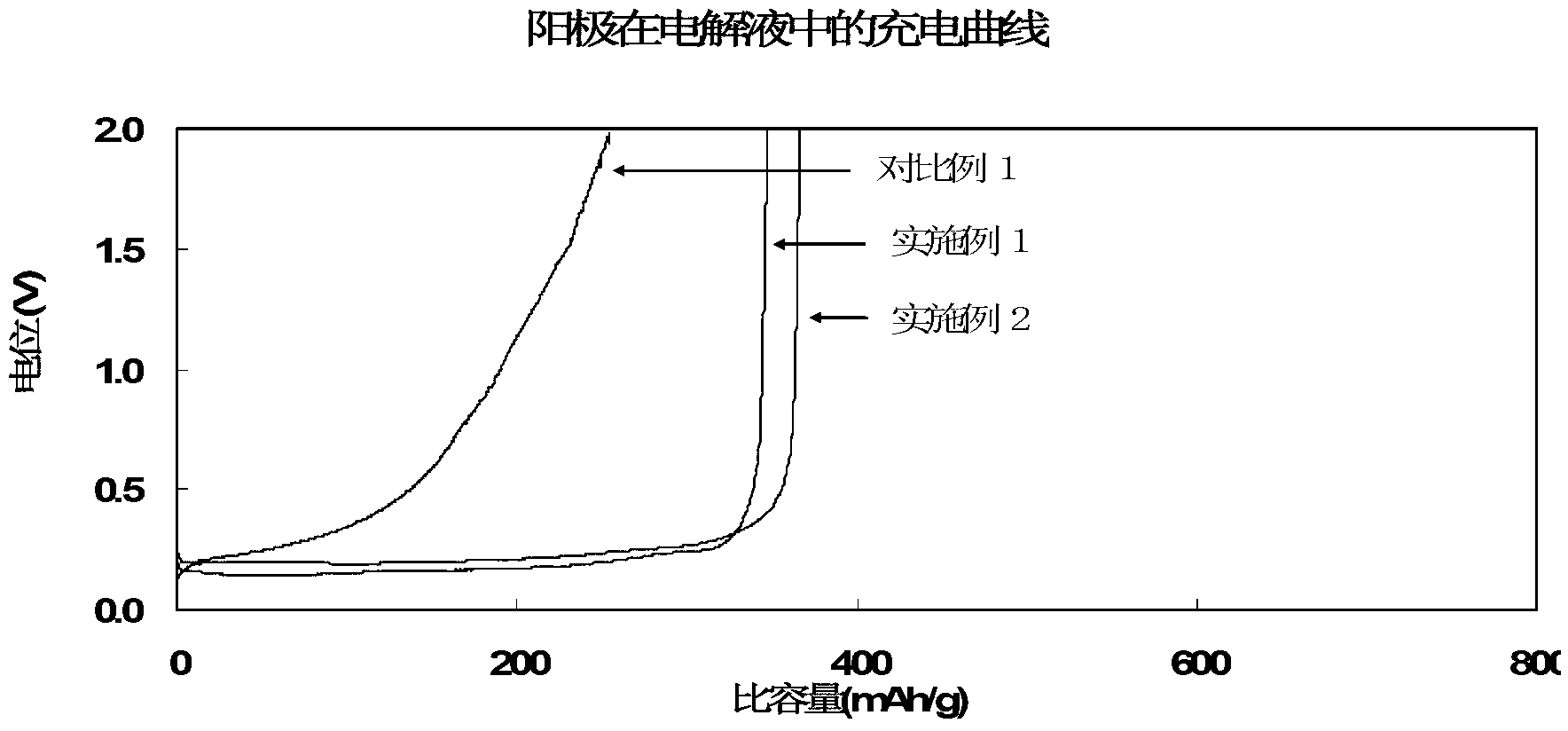
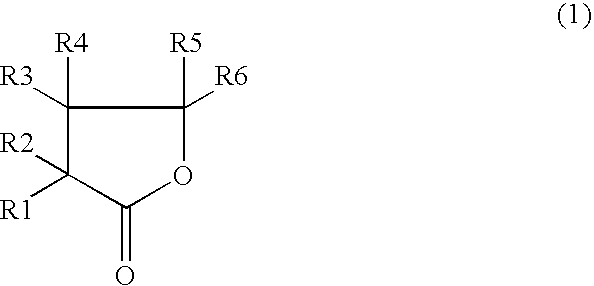
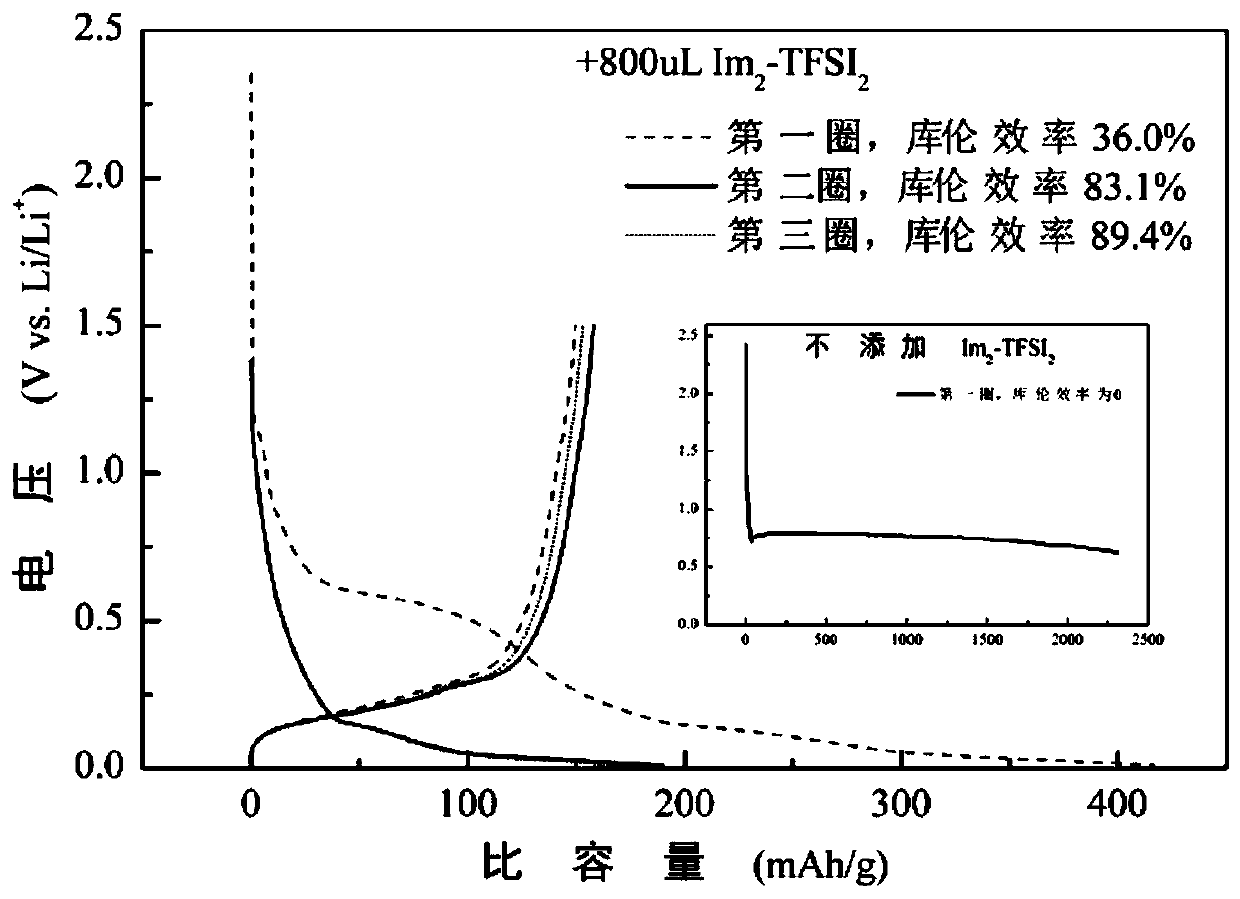
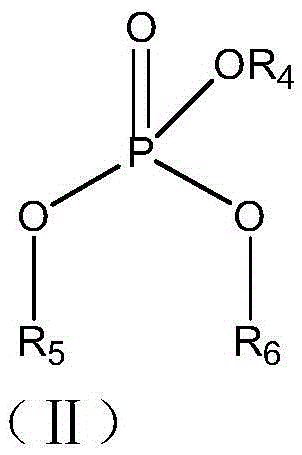
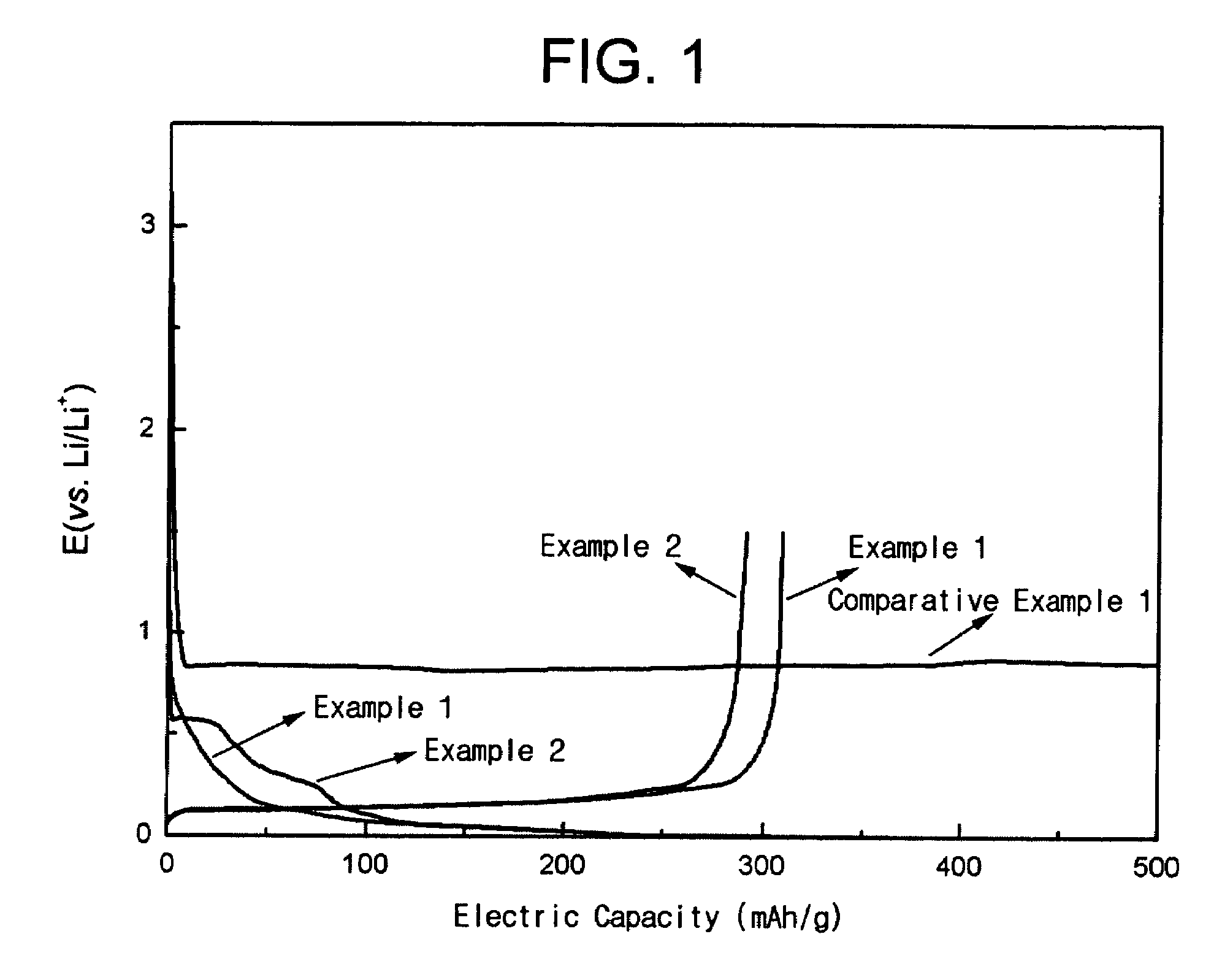
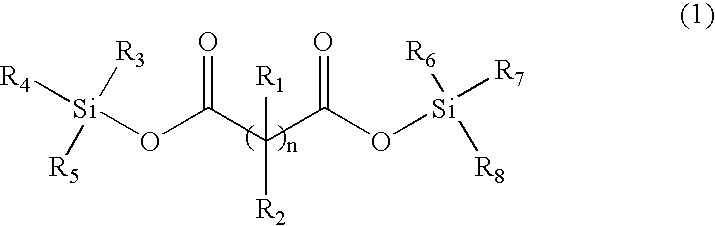
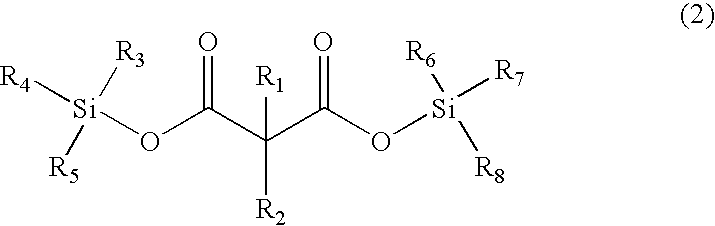
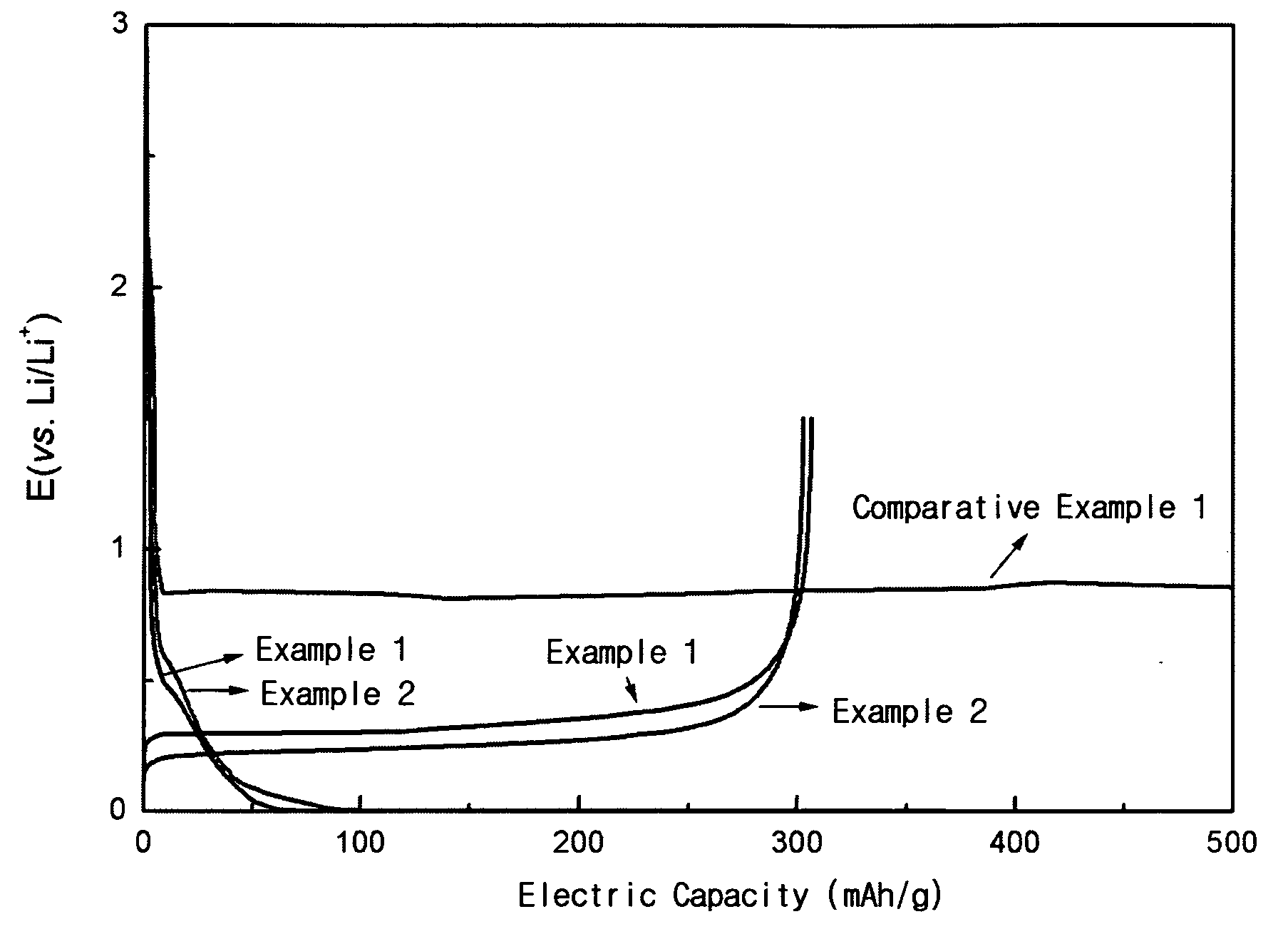
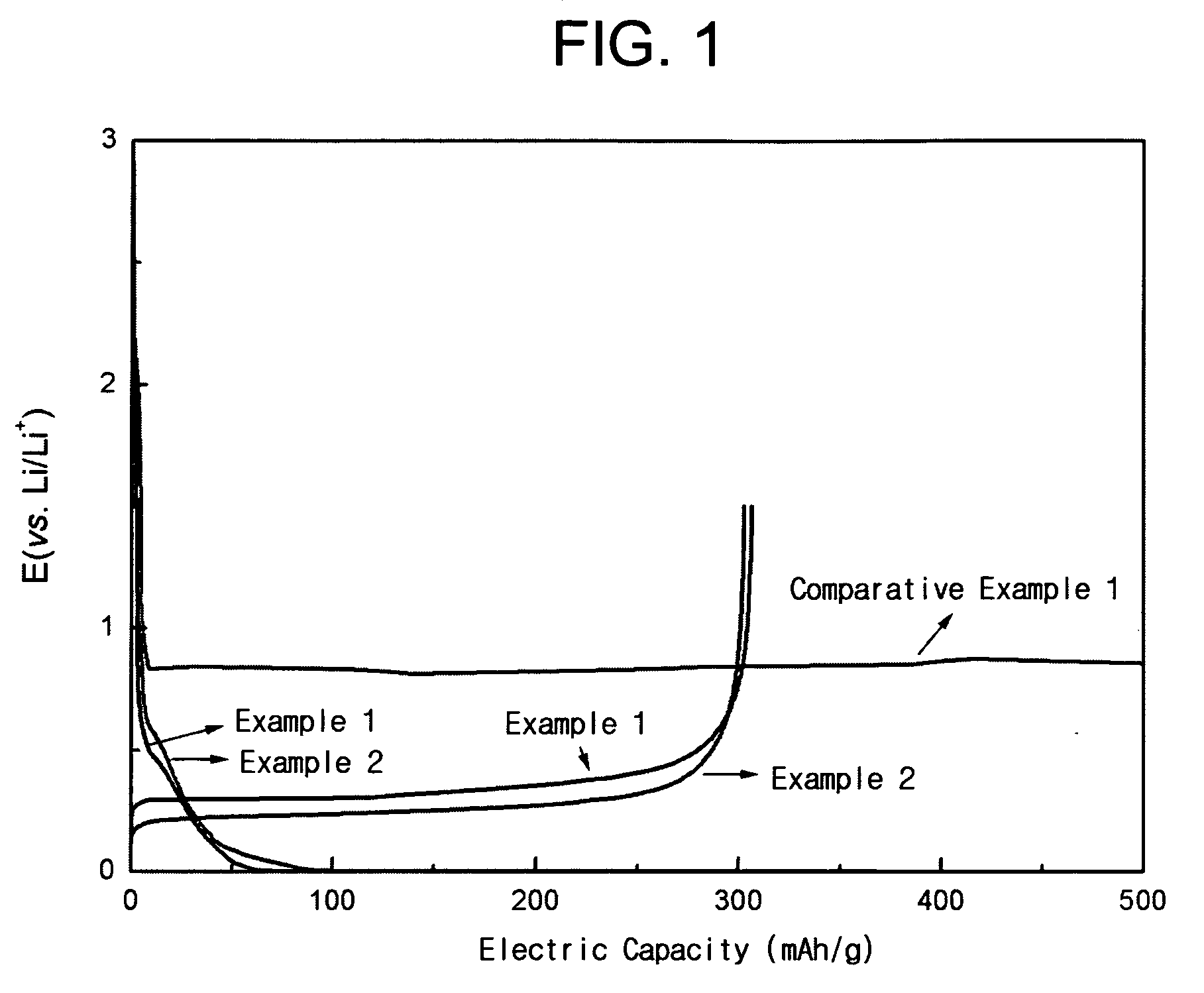
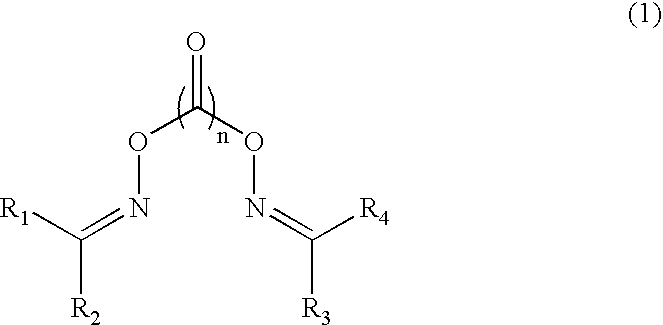
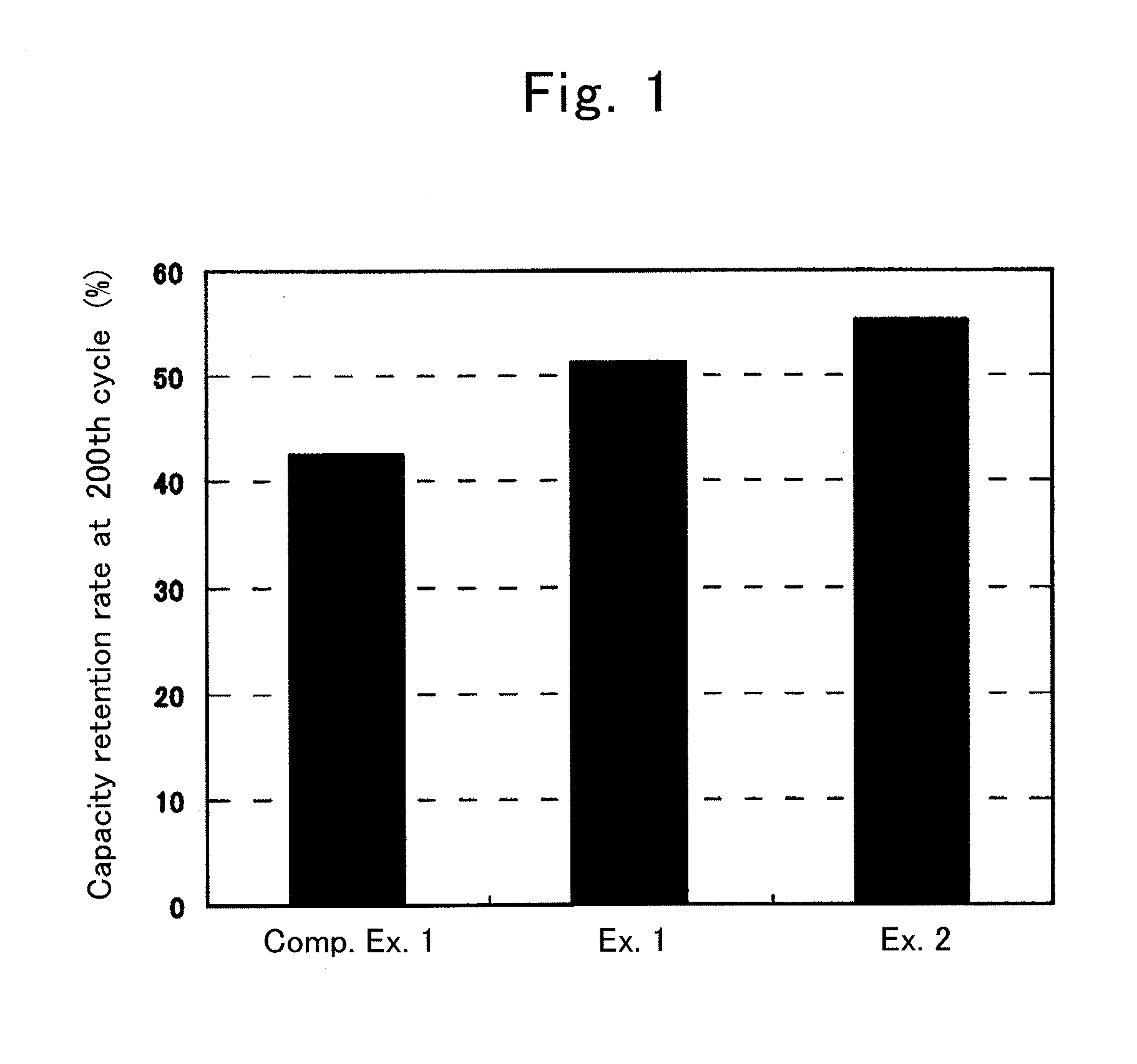
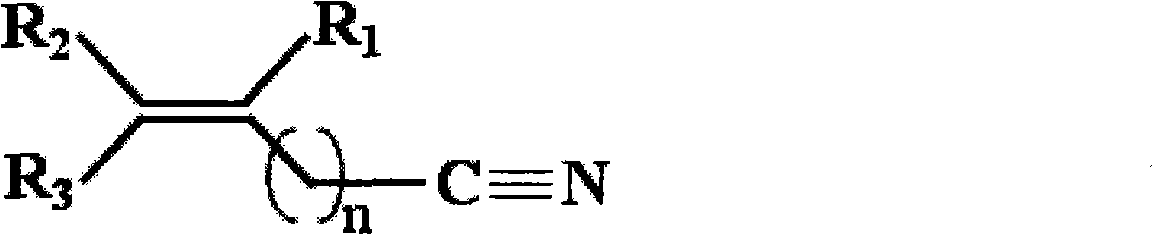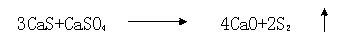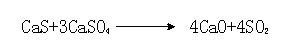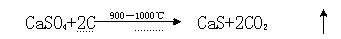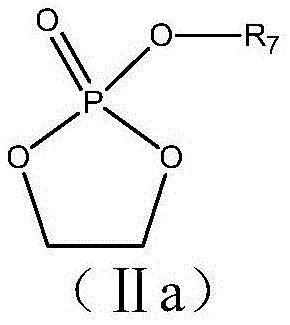Patents
Literature
108 results about "Reductive decomposition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
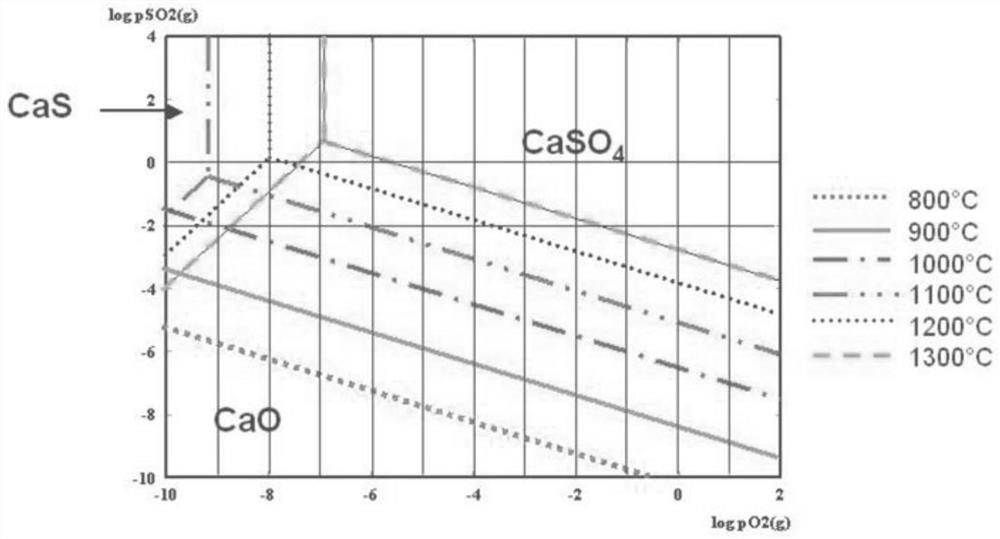
The reductive decomposition of calcium sulphate by hydrogen is used for the regeneration of calcium-based atmospheric fluidized bed combustion (AFBC) SO2 sorbents.
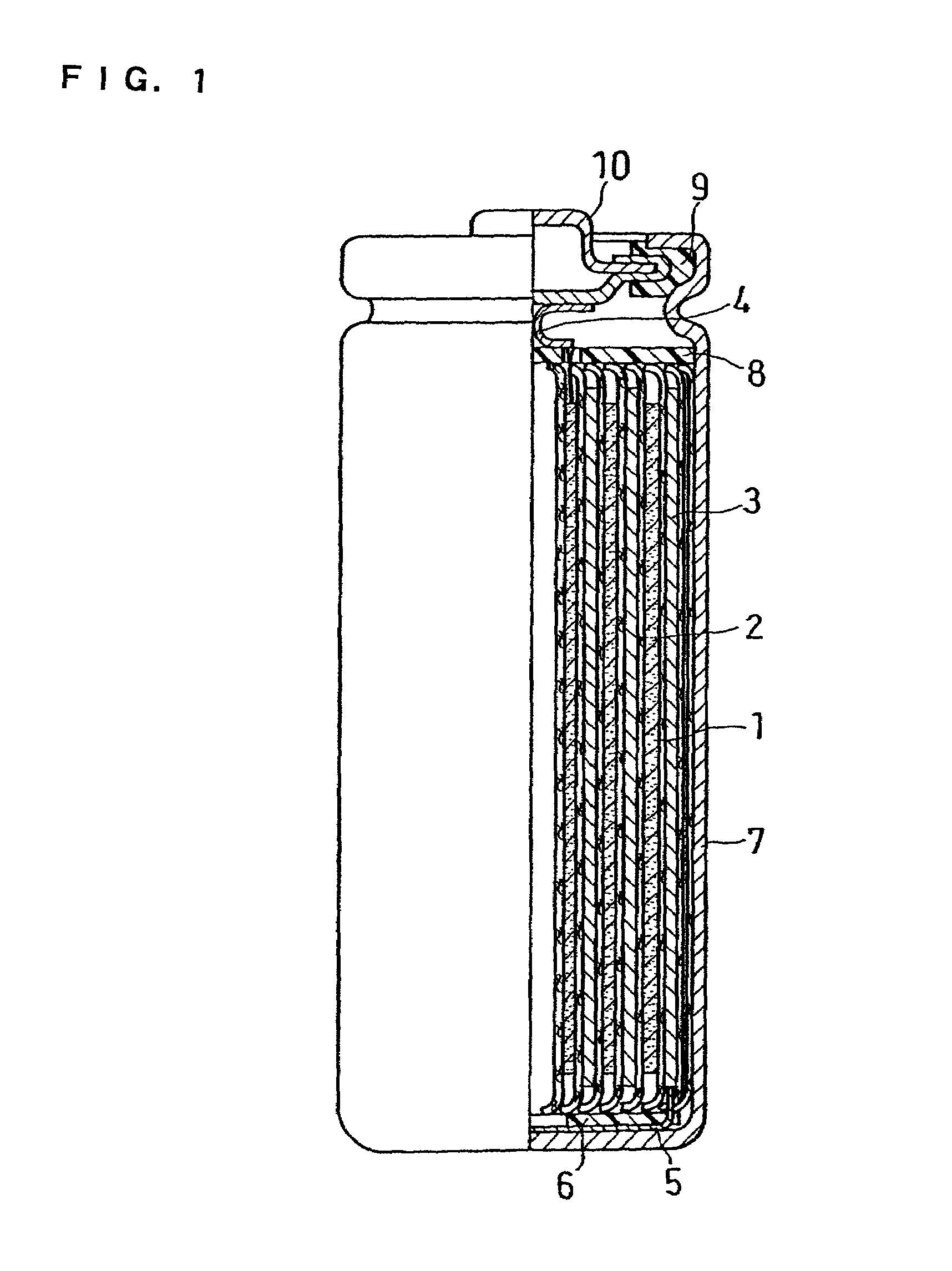
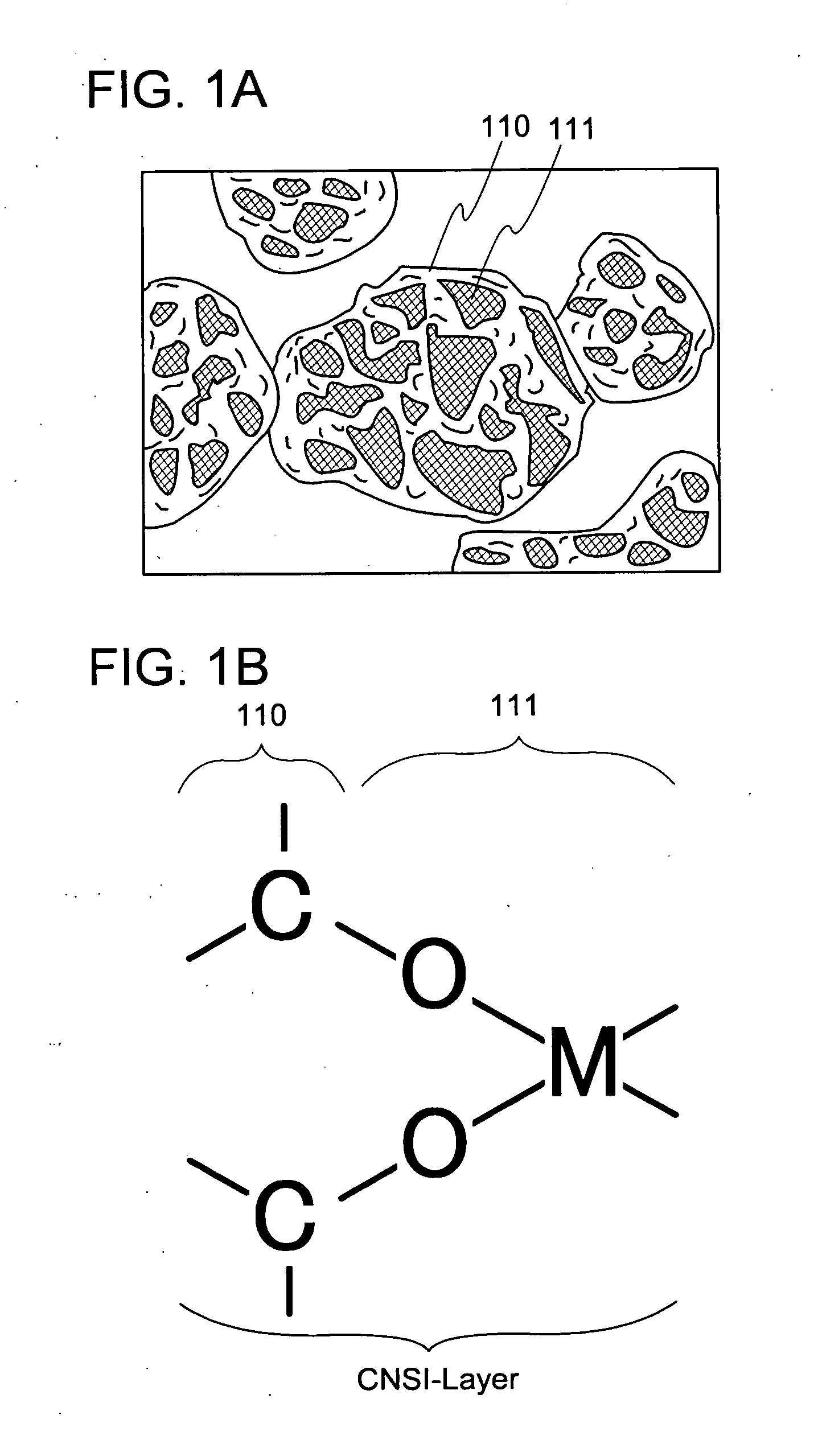
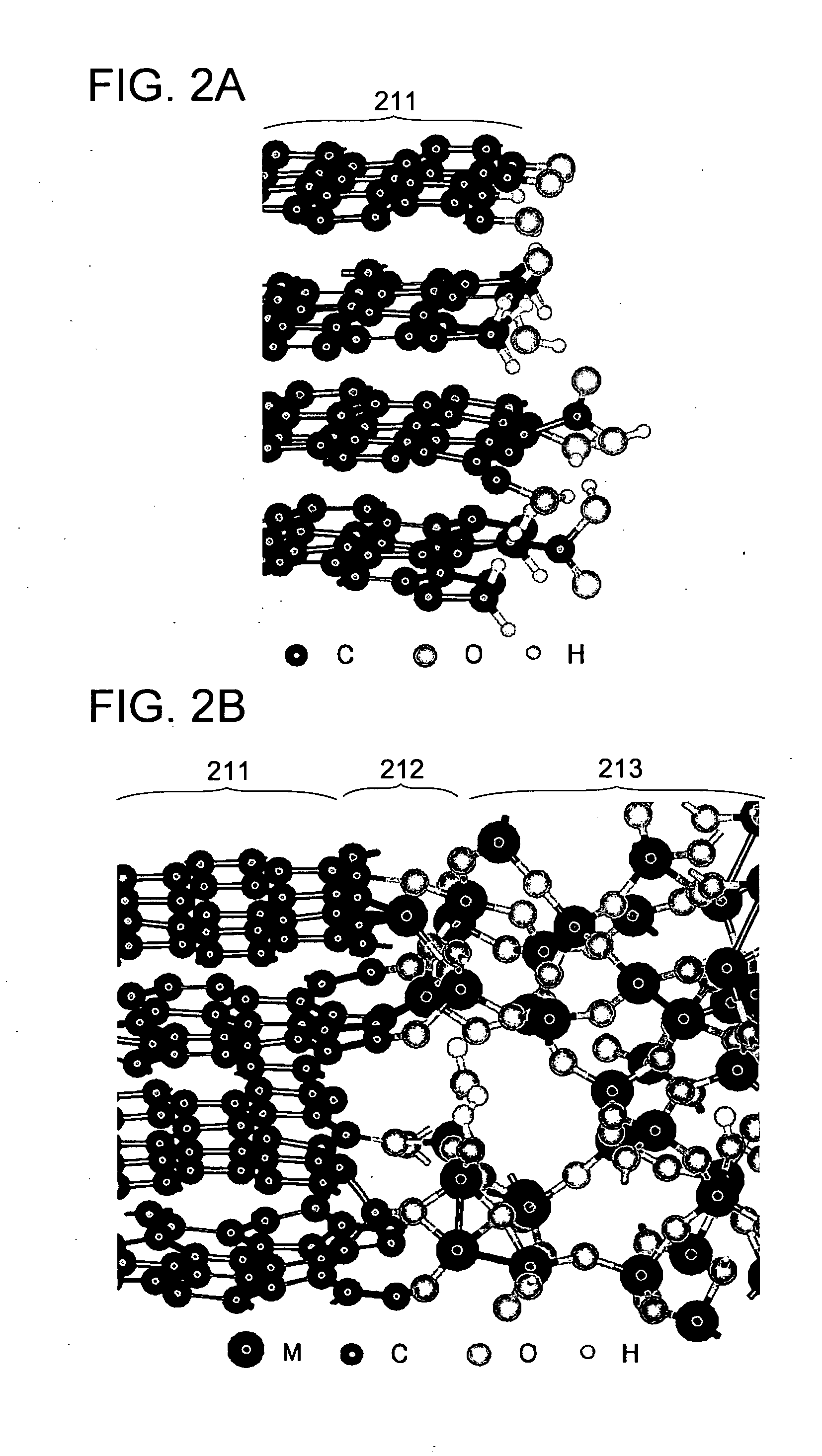
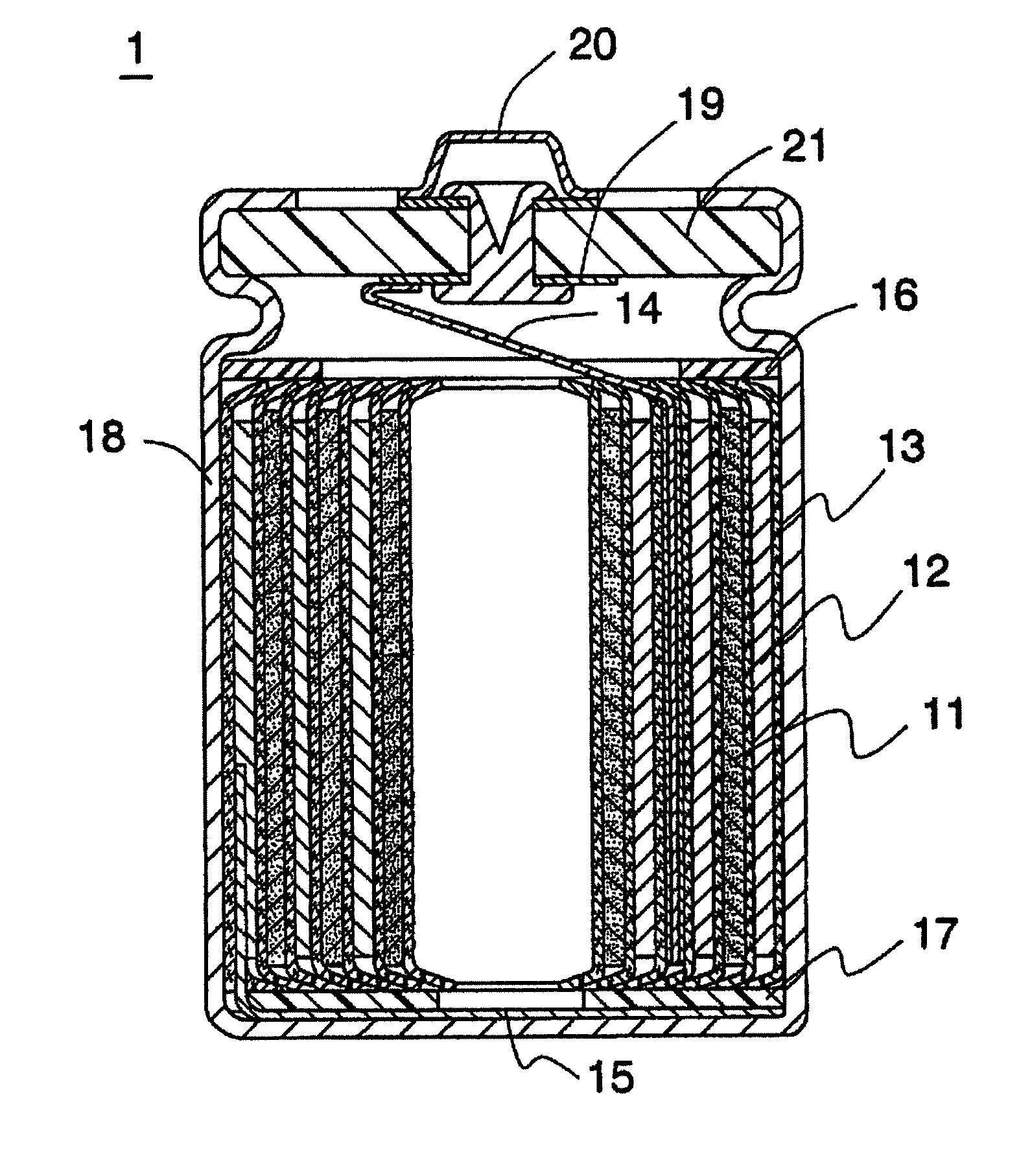
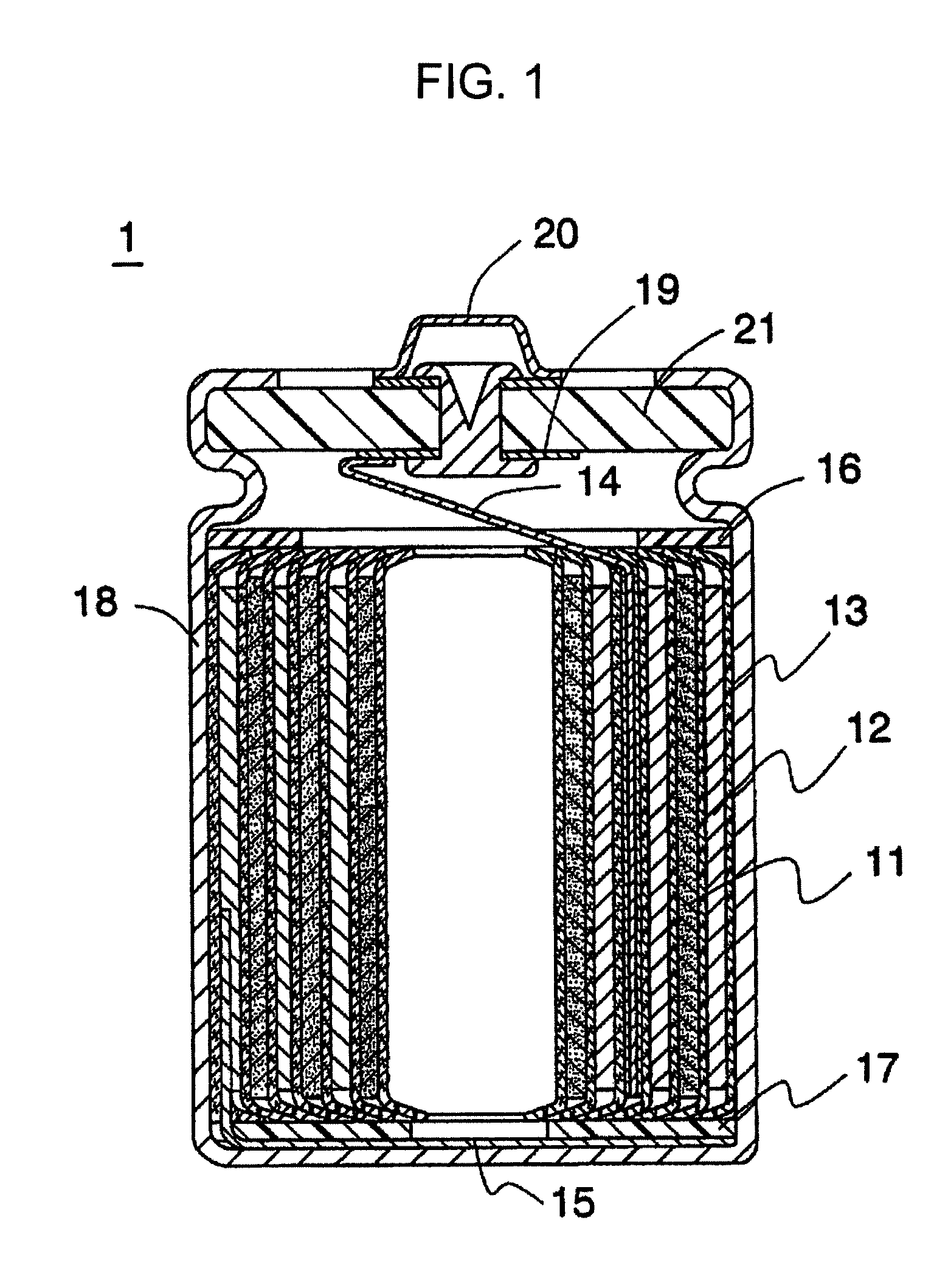
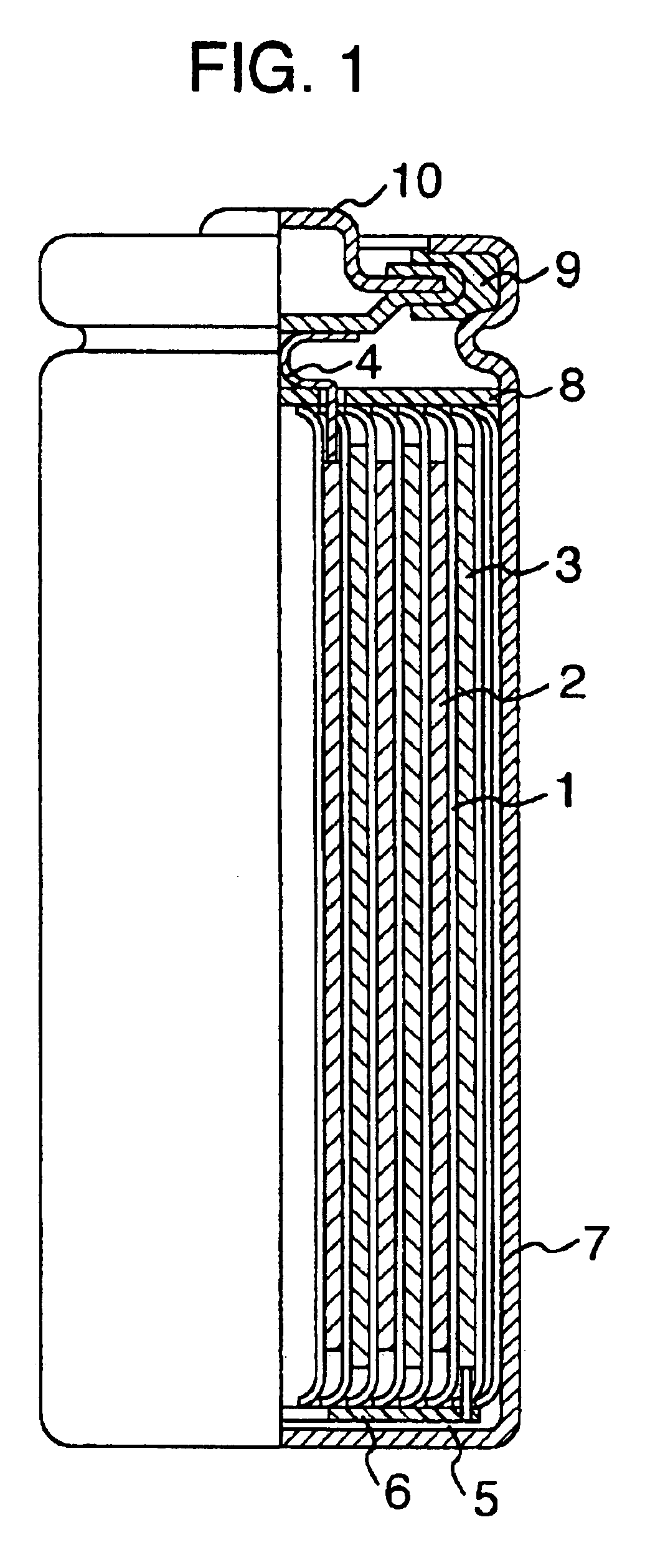
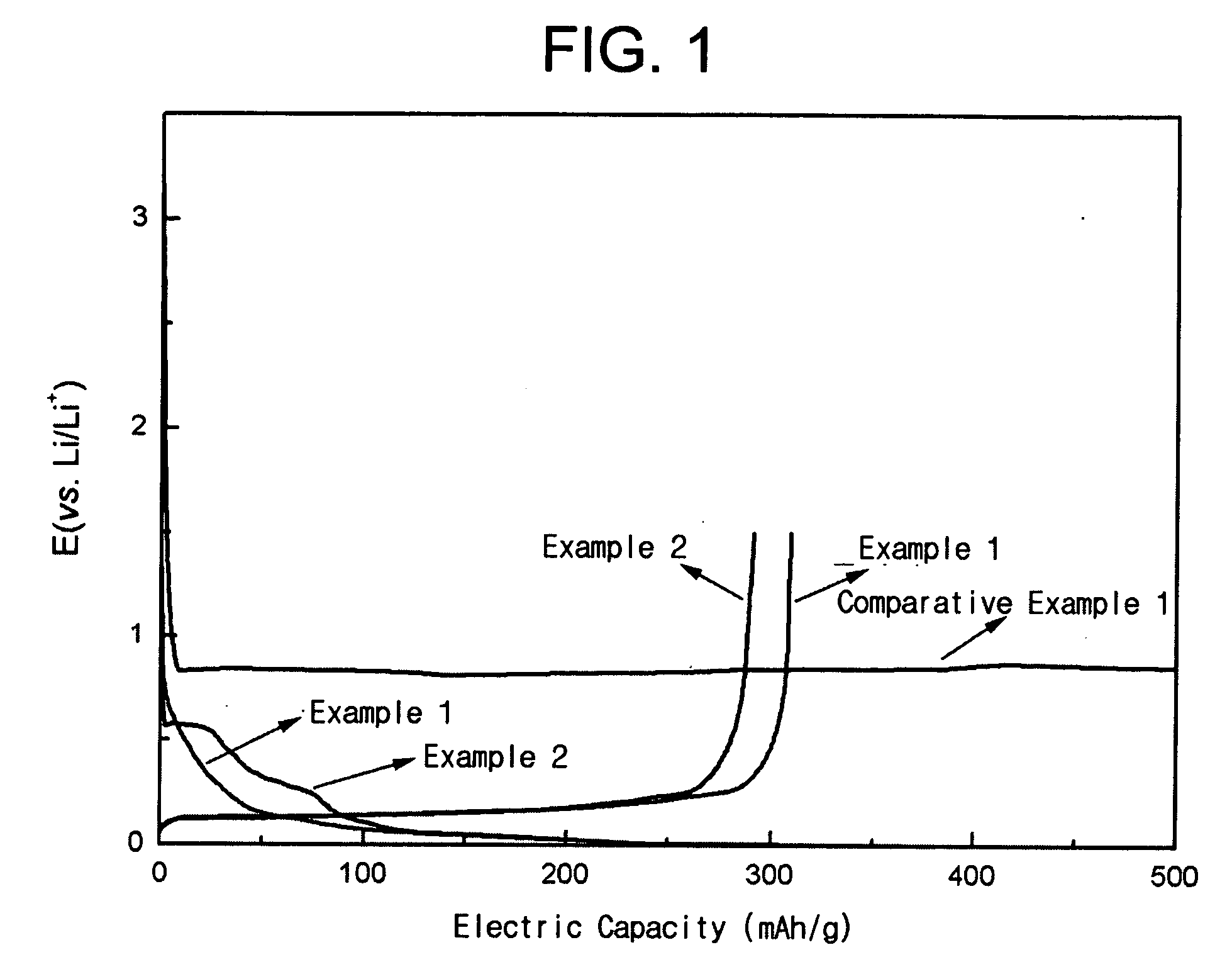
Non-aqueous electrolyte secondary battery
InactiveUS7026073B2Easy dischargeSatisfactory dischargeFuel cell auxillariesOrganic electrolyte cellsReductive decompositionCarboxylic acid
In an electrolyte of a non-aqueous electrolyte secondary battery using a cyclic carboxylic-acid ester exerting high conductivity under a low temperature condition, for suppressing reductive decomposition of the cyclic carboxylic acid ester, a cyclic carbonic acid ester having at least one carbon-carbon unsaturated bond is contained, and for suppressing excessive polymerization reaction of the cyclic carbonic acid ester having at least one carbon-carbon unsaturated bond, a cyclic carbonic acid ester having no carbon-carbon unsaturated bond is further contained.
Owner:PANASONIC CORP
Material for electrode of power storage device, power storage device, and electrical appliance
ActiveUS20140099554A1Reduce irreversible capacityReduce in quantityAluminium silicatesLiquid electrolytic capacitorsReductive decompositionSilicon
To improve the reliability of a power storage device. A granular active material including carbon is used, and a net-like structure is formed on part of a surface of the granular active material. In the net-like structure, a carbon atom included in the granular active material is bonded to a silicon atom or a metal atom through an oxygen atom. Formation of the net-like structure suppresses reductive decomposition of an electrolyte solution, leading to a reduction in irreversible capacity. A power storage device using the above active material has high cycle performance and high reliability.
Owner:SEMICON ENERGY LAB CO LTD
Nonaqueous solvent, and nonaqueous electrolyte solution and nonaqueous secondary battery that use nonaqueous solvent
InactiveUS20110236765A1Excellent cycle characteristicsInhibit gas productionNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsElectrolytic agentReductive decomposition
The nonaqueous solvent for a nonaqueous secondary battery of the present invention includes: a fluorinated cyclic carbonate having at least one fluorine in each designated location in the molecule; and a fluorinated phosphazene having at least one fluorine bound to a phosphorus atom in the phosphazene molecule and a ratio of the number of fluorine atoms to the number of phosphorus atoms being 4 / 3 or more. The fluorinated cyclic carbonate forms a good protective coat by reductive decomposition at a negative electrode, thereby improving cycle characteristics of the nonaqueous secondary battery. The fluorinated phosphazene suppresses generation of organic ions in the nonaqueous solvent, thereby reducing gas production in the nonaqueous secondary battery.
Owner:PANASONIC CORP
Lithium ion battery and electrolyte thereof
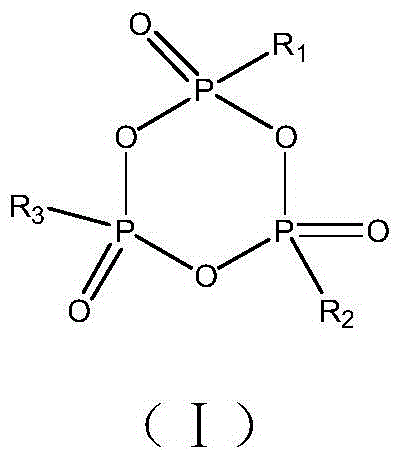
ActiveCN102738511AReduce thickness swellImprove stabilitySecondary cellsHigh temperature storageReductive decomposition
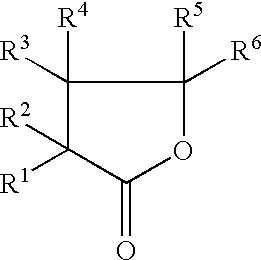
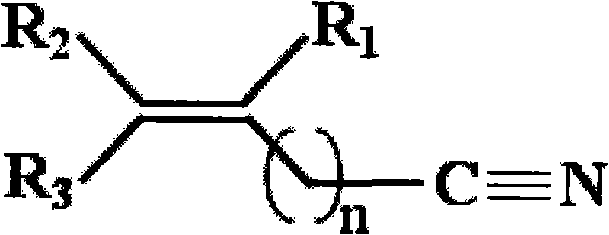
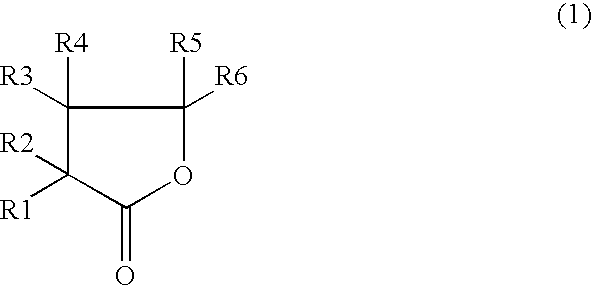
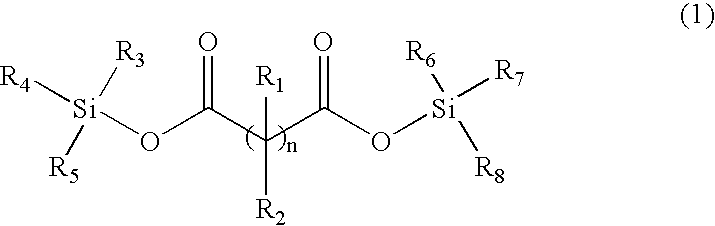
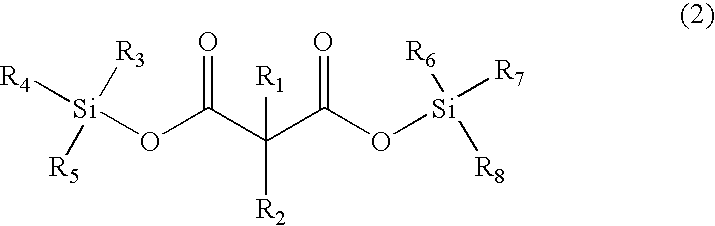
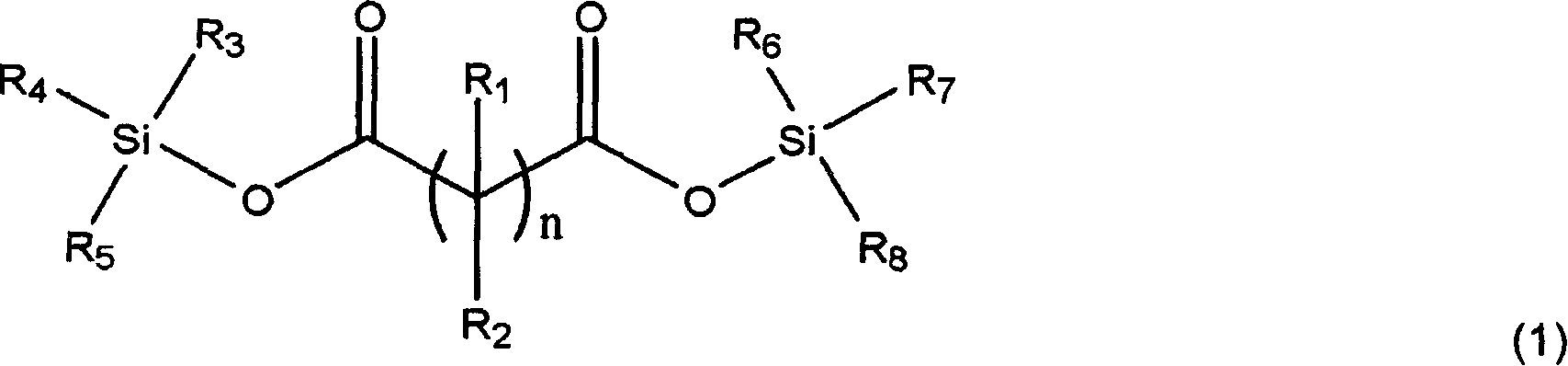
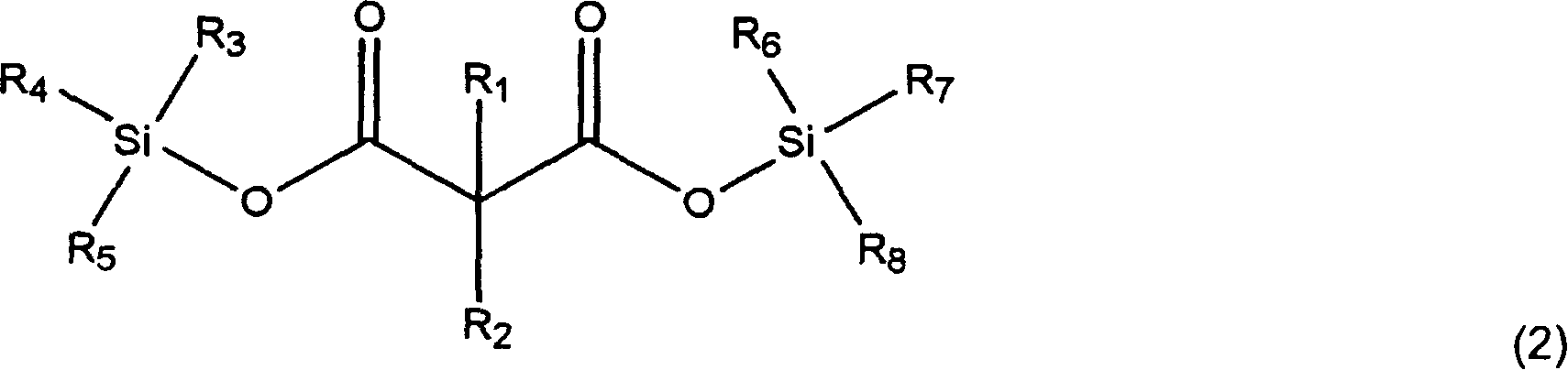
The invention, belonging to the technical field of lithium ion battery, particularly relates to a lithium ion battery electrolyte capable of improving the high temperature storage performance of a lithium ion battery, comprising a non-aqueous solvent, lithium salts dissolved in the non-aqueous solvent, and an additive which is a compound of formula (I), wherein R1, R2, and R3 are selected from hydrogen atom, alkyl containing 1-12 carbon atoms, cycloalkyl containing 3-8 carbon atoms, and aryl containing 6-12 carbon atoms, and n is an integer in the range of 0-7. The compound can effectively passivate the anode and cathode surfaces, prevent oxygenolysis of the electrolyte ingredients on the anode surface and prevent reductive decomposition on the cathode surface, thus gas generation of the battery is reduced, and the high temperature storage performance of the lithium ion battery is raised. In addition, the invention further discloses a lithium ion battery containing the electrolyte.
Owner:NINGDE AMPEREX TECH
Lithium ion battery electrolyte
ActiveCN103078139AFacilitate strippingImproving the performance of lithium insertion and extractionSecondary cellsReductive decompositionSolid state electrolyte
The invention provides a lithium ion battery electrolyte, which comprises a non-aqueous organic solvent, lithium salt soluble in the non-aqueous organic solvent, and an addition agent of a compound containing an N-sulfonyl dicarboximide group, wherein the compound containing the N-sulfonyl dicarboximide group is represented by a general formula (1); a functional group of R1 in the general formula (1) is shown in the specification, or R2 in the general formula (1) is an alkyl group of C1-C12, and a naphthenic base, an allyl group or an aryl group of C3-C8; hydrogen atoms in the R2 can be partially or wholly replaced by halogen atoms. When the compound containing the N-sulfonyl dicarboximide group is added into the lithium ion battery electrolyte, a charging process of a battery is prior to reductive decomposition of an organic solvent in the electrolyte, and a layer of solid electrolyte interphase (SEI) is better formed on a surface taking graphite as an active material, so that the problem of positive pole piece graphite stripping is effectively solved, and the performance of positive pole piece deintercalation lithium is improved.
Owner:NINGDE AMPEREX TECH
Nonaqueous electrolyte secondary cell
InactiveUS6723473B1Improve conductivityImprove discharge characteristicsNon-aqueous electrolyte accumulatorsOrganic electrolyte cellsReductive decompositionCarbonate ester
In a non-aqueous electrolyte secondary battery, a cyclic carboxylic acid ester having a high conductivity in a low-temperature environment is used as an electrolyte, and, furthermore, a cyclic carbonic acid ester having at least one carbon-carbon unsaturated bond is added thereto for the inhibition of reductive decomposition of the cyclic carboxylic acid ester.
Owner:PANASONIC CORP +1
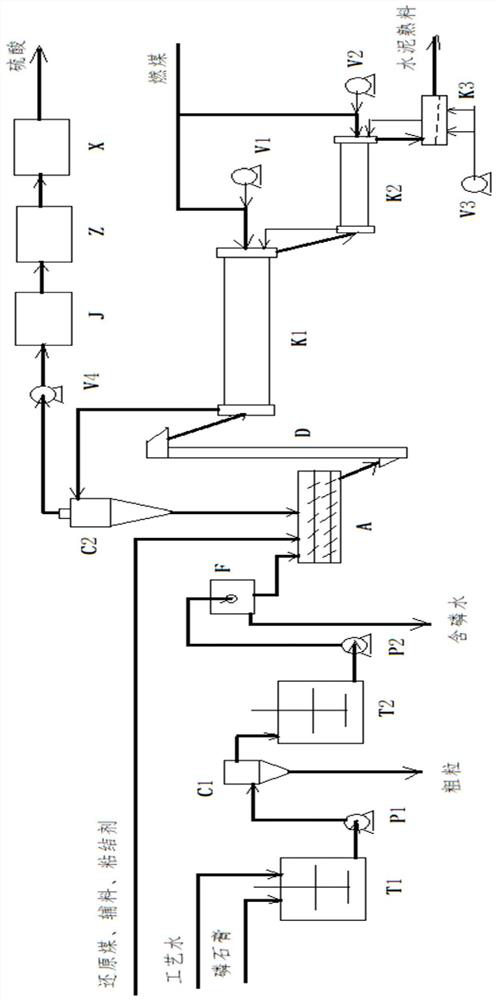
Production method for co-producing cement and sulfuric acid by using gypsum
ActiveCN103496861AReduce dosageEliminate environmental problemsSulfur compoundsEnergy inputReductive decompositionDecomposition
The invention discloses a production method for co-producing cement and sulfuric acid by using gypsum. The method comprises the following steps: carrying out hierarchical heating on a raw material A containing gypsum and coke in a hierarchical suspending pre-heater, heating and carrying out reducing decomposition on the heated gypsum raw material A in a reducing decomposition fixed bed with pulverized coal combustion, transmitting a reducing decomposition gas C generated after the reducing decomposition into the hierarchical suspending pre-heater, carrying out heat exchanging with the gypsum cement raw material A, purifying for sulfuric acid production; transmitting a material after the reducing decomposition into a cement clinker sintering fixed bed, carrying out heating sintering with pulverized coal combustion, so as to obtain a cement clinker B. Compared with the prior art, the production method for co-producing cement and sulfuric acid by using gypsum disclosed by the invention has the advantages that the dosages of reducing coal and sintering coal are reduced, the sulfur dioxide concentration in the reducing decomposition gas and the quality of a cement clinker are increased, the purposes of saving energy source, reducing production cost, enhancing production capacity, reducing investment and increasing economic benefit of a producer are realized, and the environment-protection problem of phosphor-gypsum stacking treatment is solved.
Owner:龚家竹
Organic electrolytic solution and lithium battery employing the same
ActiveUS20060172200A1Ensure reliabilityCell electrodesOrganic electrolyte cellsDischarge efficiencyReductive decomposition
An organic electrolytic solution and a lithium battery employing the same are provided. The organic electrolytic solution includes: a lithium salt; an organic solvent containing a high dielectric constant solvent and a low boiling point solvent; and a dicarboxylic acid derivative having at least one substituted silyl group. The organic electrolytic solution and the lithium battery employing the same have improved reductive decomposition stability, thereby decreasing an irreversible capacity after a first cycle and improving the charge / discharge efficiency and lifespan of the battery. The lithium battery has non-varying chemical properties at room temperature and a uniform thickness after standard charging, and thus has high reliability.
Owner:SAMSUNG SDI CO LTD
Method for producing vitriol and calcium oxide by decomposing gypsum
InactiveCN102838153ASimple processEmission reductionSulfur compoundsCalcium/strontium/barium oxides/hydroxidesReductive decompositionReducing agent
The invention provides a method for producing vitriol and calcium oxide by decomposing gypsum. The method comprises the following steps: (1) preparing materials of gypsum and coke powder based on mass ratio of 100 : (5 to 8), and grinding same; (2) preheating; (3) roasting to decompose; (4) carrying out heat exchange with the gas of a kiln, and then processing by pickling and purifying, drying, converting, and absorbing to obtain the finished product of vitriol; and (5) cooling the solid cinder to obtain the finished product of calcium oxide. According to the method provided by the invention, the coke powder serves as the main component calcium sulfate of the gypsum, which is reduced and decomposed at high temperature so as to obtain the SO2 and the solid calcium oxide; the calcium oxide produced by decomposing limestone is replaced via the calcium oxide produced by decomposing the calcium sulfate, therefore, the calcium oxide resource can be recycled, and the emission of CO2 produced by roasting the limestone at room temperature also can be reduced. The method provided by the invention is simple in material preparing and technology, easy to control the reaction atmosphere, and stable in production, has capacity of making possible the recycling of a large number of industrial by-product gypsum, is environment-friendly, and can save the resource.
Owner:XIANGFU NEW BUILDING MATERIAL HUNAN
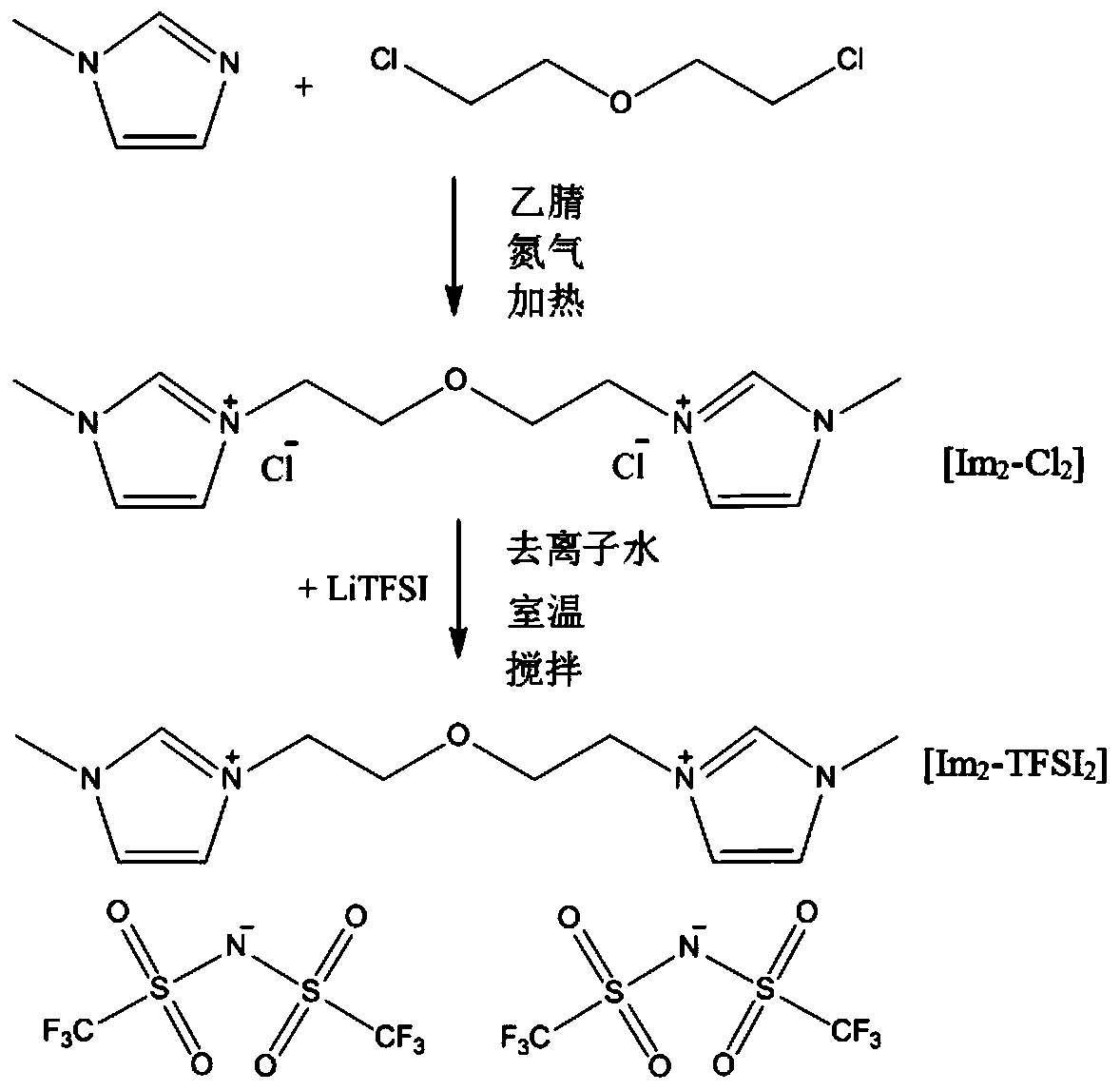
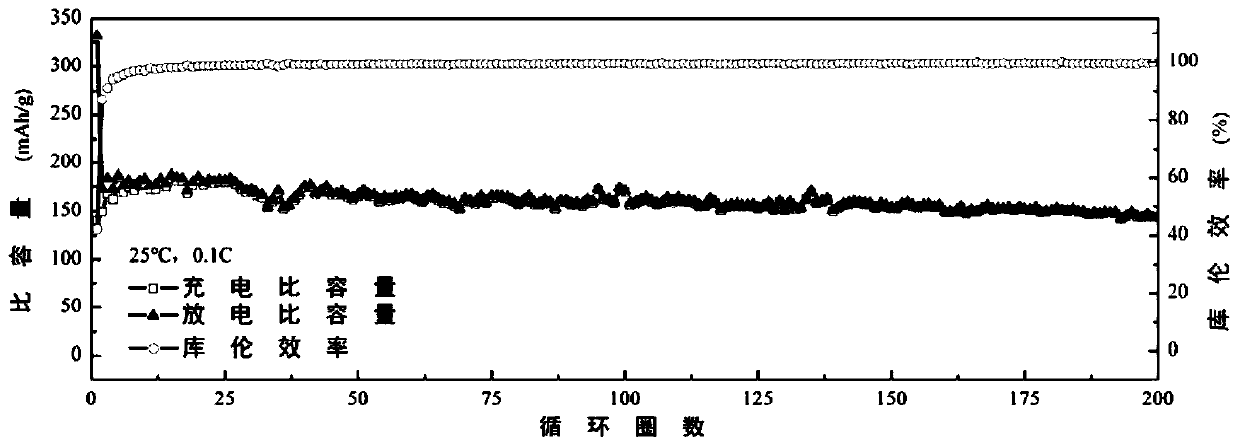
Bi-imidazole ring functional ionic liquid, preparing method thereof, electrolyte and lithium secondary battery
ActiveCN109776423AHigh thermodynamic stabilityImprove electrochemical stabilityGroup 5/15 element organic compoundsGroup 3/13 element organic compoundsReductive decompositionElectrochemical window
The invention belongs to the field of batteries, and discloses a bi-imidazole ring functional ionic liquid, a preparing method thereof, an electrolyte and a lithium secondary battery. The di-imidazolering functional ionic liquid comprises divalent cations with bi-imidazole rings and ether group functional groups and two anions. The bi-imidazole ring functional ionic liquid has higher thermodynamic stability, electrochemical stability and positive and negative electrode compatibility. Moreover, the preparing method is simple, the product is high in purity and good in hydrophobicity, the thermal decomposition temperature can reach 430 DEG C, the room-temperature conductivity can reach 10<-4>S / cm, the electrochemical window can reach 5.6V vs.Li / Li+, and the safety of the lithium secondary battery can be improved. A bi-imidazole ring ionic liquid electrolyte can effectively inhibit reductive decomposition of imidazolium cations on a graphite negative electrode specially in a graphite negative electrode system, a stable SEI membrane can be formed without addition of any low-boiling-point film-forming additive, so that the battery can be stably circulated under the conditions of normaltemperature and high temperature, and has very high practical application value.
Owner:XIAMEN UNIV
Preparing nanosize platinum-titanium alloys
InactiveUS20070131056A1Low vapor pressureReduce lossesUsing liquid separation agentCell electrodesPlatinumReductive decomposition
Nanometer sized particles containing titanium and platinum are prepared by a sonochemical process. Compounds of the metals are dissolved, suspended, or diluted in a low vapor pressure liquid medium, preferably at a sub-ambient temperature. A reducing gas is bubbled through the liquid as it is subjected to cavitation to affect the reductive decomposition of the metal compounds. Titanium and platinum are co-precipitated in very small particles.
Owner:GM GLOBAL TECH OPERATIONS LLC
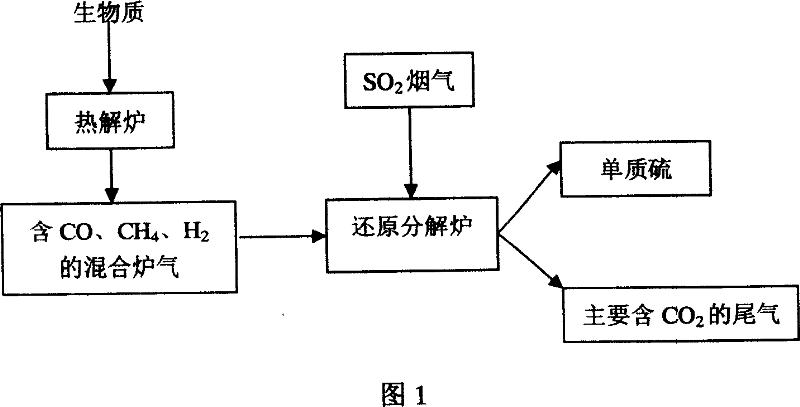
Method for deacidizing low sulfur dioxide concentration by using gas produced by biomass pyrolysis
InactiveCN101036853AEmission reductionRealize the purpose of treating waste with wasteDispersed particle separationCarburetting by solid carbonaceous material pyrolysisReductive decompositionFlue gas
The invention relates to a process for reducing SO2 to elemental sulfur in flue gas by using biomass pyrolysis gas, which belongs to the desulfurization technic field. The pyrolysis reaction for the biomass is carried out in a pyrolysis oven to generate a mixed pyrolysis gas used as desulfurizer which mainly comprises CO, CH4, and H2, the prepared catalyst is filled in a reductive decomposition oven, the mixed pyrolysis gas and the flue gas containing low-concentration SO2 are introduced into the reductive decomposition oven to have a reduction reaction at temperature of between 600DEG C and1000DEG C for 1h to 3h, the volume percentage of the SO2 gas from reductive decomposition oven can be up to 0.006% to 0.65%, and the desulfurization percent can be up to 80% to 98%, thereby realizing waste reutilization.
Owner:KUNMING UNIV OF SCI & TECH
Detection method for banned azo-dye in textile
The invention provides a detection method for banned azo-dye in a textile. The method comprises the following steps: (a) sampling and sample cutting, wherein glue and attachments on samples are cleaned as much as possible and different samples are respectively processed; (b) pretreatment and reductive decomposition of the samples; and (c) extraction and measurement of results. According to the invention, 5 mL of t-butyl methyl ether is added and the principles of liquid-liquid extraction are employed to extract amine substances in extract; a few amount of anhydrous sodium sulfate is added to remove moisture in an organic phase and to allow samples floating between two phases to settle down to the bottom, which is beneficial for absorption of the organic phase; and screening with such a method needs short time and low cost.
Owner:广州衡创测试技术服务有限公司
Nonaqueous electrolyte and lithium ion battery using same
ActiveCN105489937ARaise the reduction potentialInhibition of reductive decompositionSecondary cellsOrganic electrolytesReductive decompositionOrganic solvent
The invention belongs to the field of lithium ion batteries, and particularly relates to a nonaqueous electrolyte and a lithium ion battery using the same. The nonaqueous electrolyte is prepared from nanaqueous organic solvent, lithium salt and an additive, wherein the nonaqueous organic solvent contains at least one phosphate ester compound, and the additive contains at least one phosphonic acid cyclic anhydride compound. Due to the fact that the phosphonic acid cyclic anhydride compound has the higher reduction potential, stable SEI can be formed at the anode, reductive decomposition of phosphate ester is inhibited, and the cycling performance of the battery is improved. According to the electrolyte, by using the phosphate ester compound and the phosphonic acid cyclic anhydride compound in a combined mode, the safety performance of the battery is significantly improved, and meanwhile the cycling performance of the battery is not influenced.
Owner:NINGDE AMPEREX TECH
Organic electrolytic solution and lithium battery employing the same
ActiveUS7410731B2Cell electrodesOrganic electrolyte cellsDischarge efficiencyReductive decomposition
An organic electrolytic solution and a lithium battery employing the same are provided. The organic electrolytic solution includes: a lithium salt; an organic solvent containing a high dielectric constant solvent and a low boiling point solvent; and a dicarboxylic acid derivative having at least one substituted silyl group. The organic electrolytic solution and the lithium battery employing the same have improved reductive decomposition stability, thereby decreasing an irreversible capacity after a first cycle and improving the charge / discharge efficiency and lifespan of the battery. The lithium battery has non-varying chemical properties at room temperature and a uniform thickness after standard charging, and thus has high reliability.
Owner:SAMSUNG SDI CO LTD
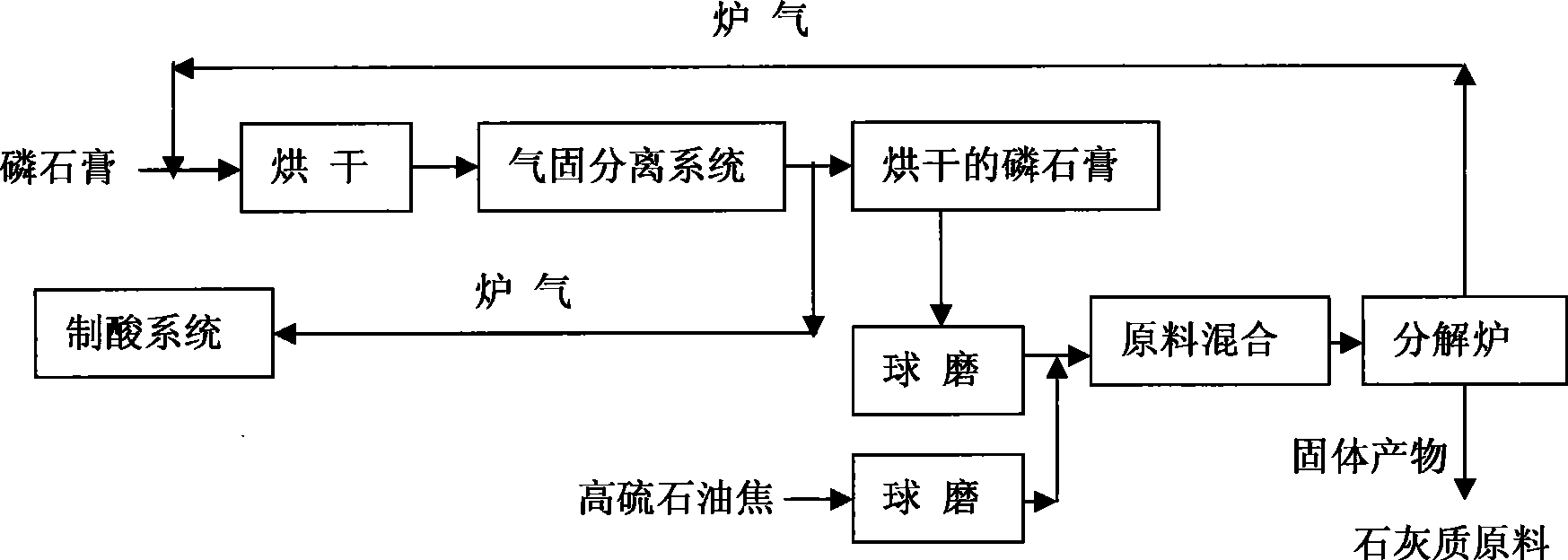
Method for preparing lime raw material and SO2 by decomposing phosphogypsum from high sulphur refinery coke
InactiveCN101486480AStable concentrationIncrease contentSolid waste disposalSulfur compoundsReductive decompositionDecomposition
The invention discloses a method for preparing a calcium carbonate raw material and SO2 by utilizing high-sulfur petroleum coke to decompose phosphogypsum, i.e., the high-sulfur petroleum coke with the carbon mass fraction higher than 80 percent and the sulfur mass fraction higher than 2 percent to 6 percent is used for reducing and decomposing the phosphogypsum to prepare burner gas with the SO2 volume percentage higher than or equal to 15 percent. The burner gas can be directly used as qualified virgin gas for the two-conversion and two-absorption acid-making technology so as to produce the solid product, CaO, with the mass fraction of higher than 50 percent, and can also be directly used as a chemical raw material, for instance, for making cement. No waste is generated in the process, the decomposition rate of the phosphogypsum is higher than or equal to 95 percent, and the desulphurization degree is higher than or equal to 90 percent. By utilizing the technology for decomposing the phosphogypsum, pretreatment is not required and the concentration of SO2 is stable in the burner gas. The content and the thermal value of carbon in the high-sulfur petroleum coke are comparatively high, and the contents of nitrogen and sulfur are higher than that of other fuels, thereby reducing reaction temperature, energy consumption and production cost, simultaneously, environment pollution is not generated and waste is changed into valuable. The utilization of components of the phosphogypsum is maximized during the technical process so as to solve the problem of realizing the harmlessness and sales of the phosphogypsum as 'dangerous waste'.
Owner:KUNMING UNIV OF SCI & TECH
Method for reduction and decomposition of phosphogypsum with yellow phosphorus tail gas
InactiveCN101492177AEmission reductionRealize comprehensive utilizationCarbon compoundsSulfur compoundsReductive decompositionFurnace temperature
The invention relates to a method for reducing and decomposing ardealite by tail gas of phosphor. The method comprises that: the naturally dried ardealite is put into a reductive decomposition furnace and filled with the tail gas of phosphor for reaction, and the furnace temperature is controlled to be between 700 and 1,000 DEG C, and the reaction time is between 10 and 50 minutes. The obtained furnace gas in which the content of the sulfur dioxide in percentage by volume is more than or equal to 15 percent is used as a material gas for the double-rotation double-suction acid preparation process; and the content of CaO, namely a solid product is more than or equal to 70 weight percent and is used as a cement material.
Owner:KUNMING UNIV OF SCI & TECH
Organic electrolytic solution and lithium battery employing the same
ActiveUS7279249B2Deterioration can be suppressedConductive materialOrganic electrolyte cellsDischarge efficiencyReductive decomposition
An organic electrolytic solution containing an organic solvent and a compound that contains an anionic polymerization monomer with an added component capable of being chelated with a lithium metal cation. A lithium battery may utilize the organic electrolytic solution. The lithium battery may have improved stability to reductive decomposition, reduced first cycle irreversible capacity, and improved charging / discharging efficiency and lifespan. Moreover, reliability of the battery may be improved because the battery, after formation and standard charging at room temperature, may not swell beyond a predetermined thickness. Even when the lithium battery swells significantly at a high temperature, the capacity of the lithium battery may be high enough for practical applications due to its recovery capacity.
Owner:SAMSUNG SDI CO LTD
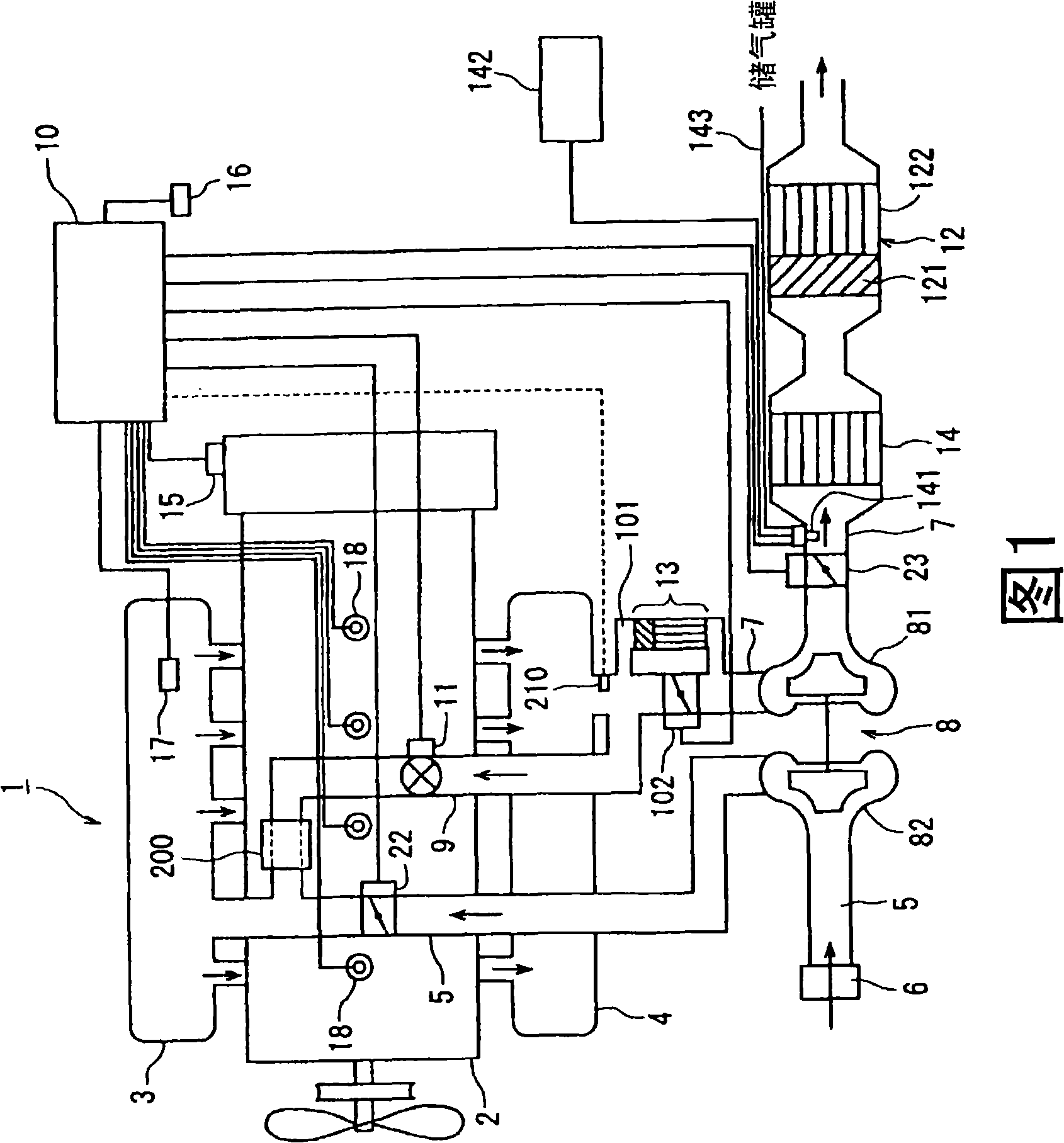
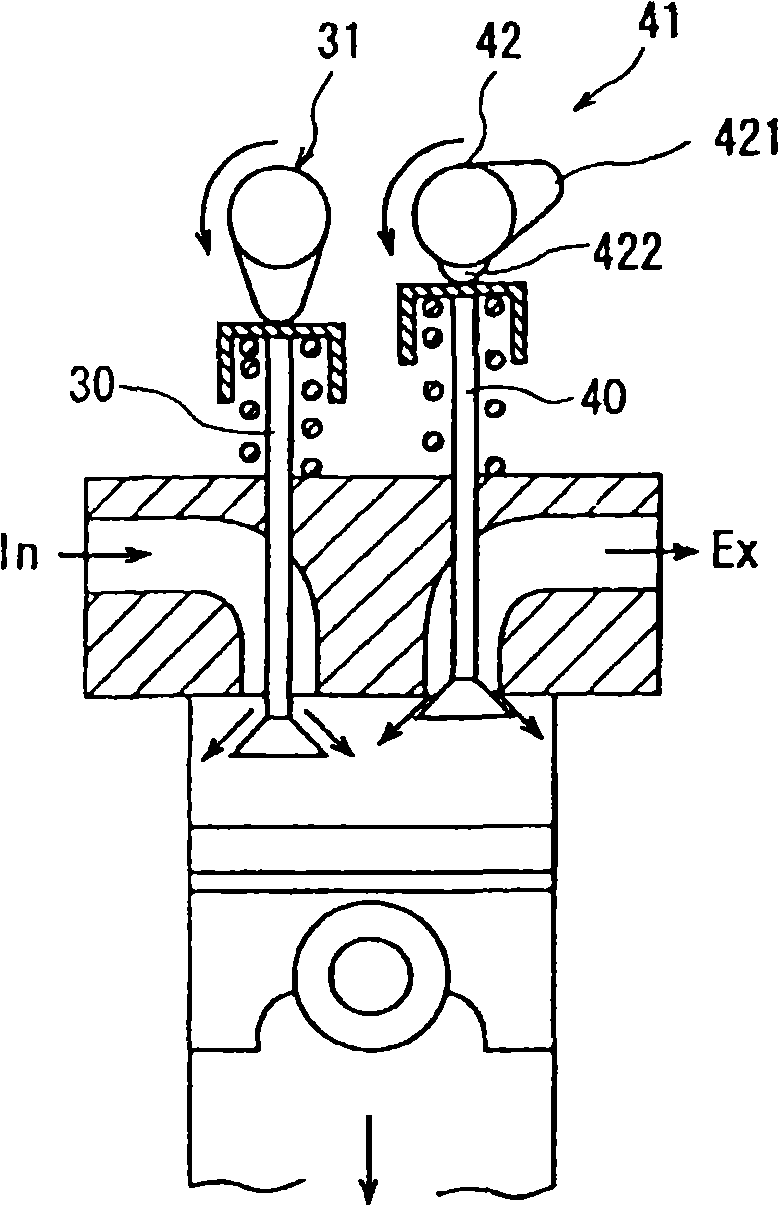
Exhaust gas purifier for diesel engine
InactiveCN101283169AReduce excess airPoor fuel economyGas treatmentElectrical controlReductive decompositionExhaust fumes
This invention provides an exhaust gas purifier for a diesel engine provided with a continuous regeneration-type DPF using a catalyst. A by-pass passage is provided in an exhaust passage (7) in a diesel engine provided with a continuous regeneration-type DPF (12), and a small-capacity second continuous regeneration-type DPF (13) is installed. In a low-exhaust gas temperature region, PM is collected by the second continuous regeneration-type DPF (13). In this case, premixing combustion in which fuel injection timing for injecting a fuel into a cylinder is hastened is carried out to reduce the amount of PM produced. Accordingly, satisfactory collection can be carried out even when the capacity of the second continuous regeneration-type DPF (13) is small. Further, since the production of NOx is suppressed, a cooled exhaust gas is recirculated into a cylinder. Further, a urea-added SCR catalyst is installed in the exhaust passage (7) for reductive decomposition of NOx contained in the exhaust gas to further reduce NOx.
Owner:ISUZU MOTORS LTD
Organic electrolytic solution and lithium battery employing the same
ActiveUS20060147807A1Electrolytic capacitorsCell electrodesReductive decompositionDischarge efficiency
An organic electrolytic solution and a lithium battery employing the same are provided. The organic electrolytic solution includes: a lithium salt; an organic solvent containing a high dielectric constant solvent and a low boiling point solvent; and a carbonate or oxalate derivative having at least one substituted or unsubstituted cyano group. The organic electrolytic solution and the lithium battery employing the same have improved reductive decomposition stability, thereby decreasing an irreversible capacity after a first cycle and improving the charge / discharge efficiency and lifespan of the battery. The lithium battery has non-varying chemical properties at room temperature and a uniform thickness after standard charging, and thus has high reliability.
Owner:SAMSUNG SDI CO LTD
Method for decomposing phosphogypsum with enclosed calcium carbide burner gas reduction
InactiveCN101492176ALower decomposition temperatureTo achieve the purpose of saving energy and reducing consumptionCarbon compoundsChemical industryHigh concentrationReductive decomposition
The invention relates to a method for reducing and decomposing ardealite by furnace gas of a closed calcium carbide furnace. The technical problems needed to be solved comprise that: carbon monoxide in the furnace gas of the closed calcium carbide furnace is used as a reducer to reduce and decompose the ardealite, the decomposition temperature of the ardealite is controlled to be between 750 and 850 DEG C, the reaction time is between 10 and 50 minutes, sulfur dioxide with high concentration and qualified cement materials are obtained, the decomposition rate of the ardealite is more than or equal to 95 percent, and the desulphurization rate is more than or equal to 90 percent. The decomposition temperature of the ardealite is greatly reduced, thereby achieving the aims of saving energy and reducing consumption. The method develops potential carbon-sulfur resources, and realizes the comprehensive utilization of the ardealite and the furnace gas of the closed calcium carbide furnace; and simultaneously, the obtained calcium oxide can be used as a raw material to produce high quality cement, and the sulfur dioxide can be used as a material gas to produce acid.
Owner:KUNMING UNIV OF SCI & TECH
Negative electrode for nonaqueous secondary battery, method for producing the same, and nonaqueous secondary battery
ActiveUS20140242458A1Small thicknessUniform refinementFinal product manufactureActive material electrodesReductive decompositionZeta potential
Provided is a negative electrode for a nonaqueous secondary battery which achieves both suppression of reductive decomposition of an electrolyte or an electrolytic solution and suppression of an increase in resistance. Also provided are a method for producing such a negative electrode and a nonaqueous secondary battery employing such a negative electrode. A polymer coating layer is formed so as to coat at least part of surfaces of negative electrode active material particles containing silicon oxide (SiOx; 0.5≦x≦1.6). The polymer coating layer contains a cationic polymer having a positive zeta potential under neutral conditions. Since silicon oxide has a negative zeta potential, a thin uniform coating layer can be formed owing to Coulomb's force.
Owner:TOYOTA IND CORP
Production method for producing cement and sulfuric acid from phosphogypsum
InactiveCN112694067AImprove product qualityEliminate the environmental problems of stackingSulfur compoundsCeramic production plantsReductive decompositionDust control
The invention discloses a production method for producing cement and sulfuric acid by using phosphogypsum. The method comprises the following steps: carrying out pretreatment purification on phosphogypsum to reduce insoluble phosphorus, water-soluble phosphorus impurities and most free water in the phosphogypsum, kneading with a reducing agent, granulating, and directly feeding the material into a reductive decomposition integrated rotary kiln with fluidization preheating function; controlling a gas phase atmosphere to perform step-by-step heating, drying, dehydration and reductive decomposition under the combustion of the pulverized coal; sulfur dioxide gas generated after reductive decomposition is used for producing sulfuric acid after being subjected to dust removal and purification; and the reduced and decomposed material enters an oxidation calcining kiln for cement clinker sintering, and the gas-phase atmosphere is controlled under the combustion of pulverized coal for heating, mineralizing and sintering to obtain the cement clinker. Compared with the prior art, the production method for producing cement and co-producing sulfuric acid by using phosphogypsum has the advantages that the coal consumption for reduction and firing is reduced, the production efficiency and the product quality are improved, the device investment is saved, the economic benefit of producers is increased, and the environmental protection problem of phosphogypsum stacking treatment is eliminated.
Owner:CHENGDU QIANLIJIN TECHCAL INNOVATION CO LTD
Organic electrolytic solution and lithium battery employing the same
An organic electrolytic solution and a lithium battery employing the same are provided. The organic electrolytic solution includes: a lithium salt; an organic solvent containing a high dielectric constant solvent and a low boiling point solvent; and a dicarboxylic acid derivative having at least one substituted silyl group. The organic electrolytic solution and the lithium battery employing the same have improved reductive decomposition stability, thereby decreasing an irreversible capacity after a first cycle and improving the charge / discharge efficiency and lifespan of the battery. The lithium battery has non-varying chemical properties at room temperature and a uniform thickness after standard charging, and thus has high reliability.
Owner:SAMSUNG SDI CO LTD
Electrochemical energy storage device
InactiveUS20080254363A1Shorten cycle lifeLow efficiencyAlkaline accumulatorsHybrid capacitor electrolytesElectrode potentialReductive decomposition
The present invention provides an electrochemical energy storage device comprising a positive electrode, a negative electrode, and a non-aqueous electrolytic solution containing an ammonium salt, wherein the negative electrode potential upon completion of charging is set to less than 1.8 V and 0.1 V or more relative to a lithium reference in which high electric capacity is obtained and a reductive decomposition reaction of the ammonium salt on the negative electrode is avoided, and thus efficiency upon every charging and discharging cycle is improved, resulting in a long cycle life.
Owner:PANASONIC CORP
Sulfide solid electrolyte
ActiveCN104604013ALow reduction and decomposition potentialSolid electrolytesSulfide conductorsReductive decompositionOctahedron
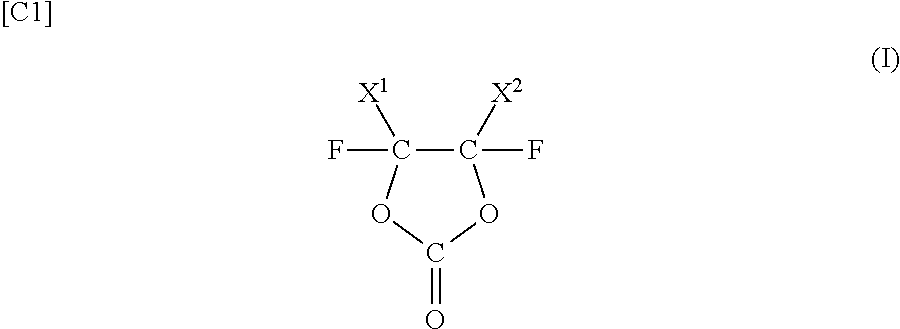
The main purpose of the present invention is to provide a sulfide solid electrolyte that allows reductive decomposition potential to be reduced to a greater extent than a conventional LGPS sulfide solid electrolyte. The present invention is a sulfide solid electrolyte containing Li, Al, Ge, P and S, such that M0 = M2 / M1 where 0<M0<0.323 (M1: molar fraction of P content; M2: molar fraction of Al content). The crystal structure of the sulfide solid electrolyte is provided with an octahedron (O) formed by Li and S, a tetrahedron (T1) formed by S and X1, and a tetrahedron (T2) formed by S and X2 (X1, X2: elements selected from the group consisting of P, Ge and Al). The octahedron (O) and the tetrahedron (T1) share a ridge, and the octahedron (O) and the tetrahedron (T2) share a vertex.
Owner:TOYOTA JIDOSHA KK +1
Sulfuryl electrolyte for high-energy-density and high-safety lithium ion battery and preparation method of Sulfuryl electrolyte
InactiveCN107959050AImprove cycle performanceImprove cycle stabilitySecondary cellsReductive decompositionHigh concentration
The invention discloses a sulfuryl electrolyte for a high-energy-density and high-safety lithium ion battery and a preparation method of the sulfuryl electrolyte, and belongs to the technical field oflithium ion batteries. Conductive lithium salt is added into common electrolyte to prepare the sulfuryl electrolyte, the concentration of the conductive lithium salt is 1.5-2.5 times that of the common electrolyte, the common electrolyte comprises sulfone solvents, linear carbonic ester solvents and the conductive lithium salt, and the conductive lithium salt is fluorine-containing lithium salt.According to the sulfuryl electrolyte, the circulation performance of the sulfuryl electrolyte is improved by the aid of high-concentration lithium salt, a graphite negative electrode surface film isoptimized in the discharging process under the condition of the high-concentration lithium salt, surface activity of an electrode is restrained, so that further contact between the electrolyte and anactive substance of the electrode is restrained, reductive decomposition of sulfone solvents of the electrolyte on the surface of the electrode is decreased, circulation stability of the lithium ion batteries under the sulfuryl electrolyte is improved, and safety, service life, energy density and specific discharge capacity are improved.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Bimetallic catalyst for high nitrate nitrogen reduction and selectivity and manufacturing methods thereof
InactiveUS20160347633A1Improve efficiencyHigh selectivityHeat treatmentsWater treatment compoundsNitrate nitrogenIron oxyhydroxide
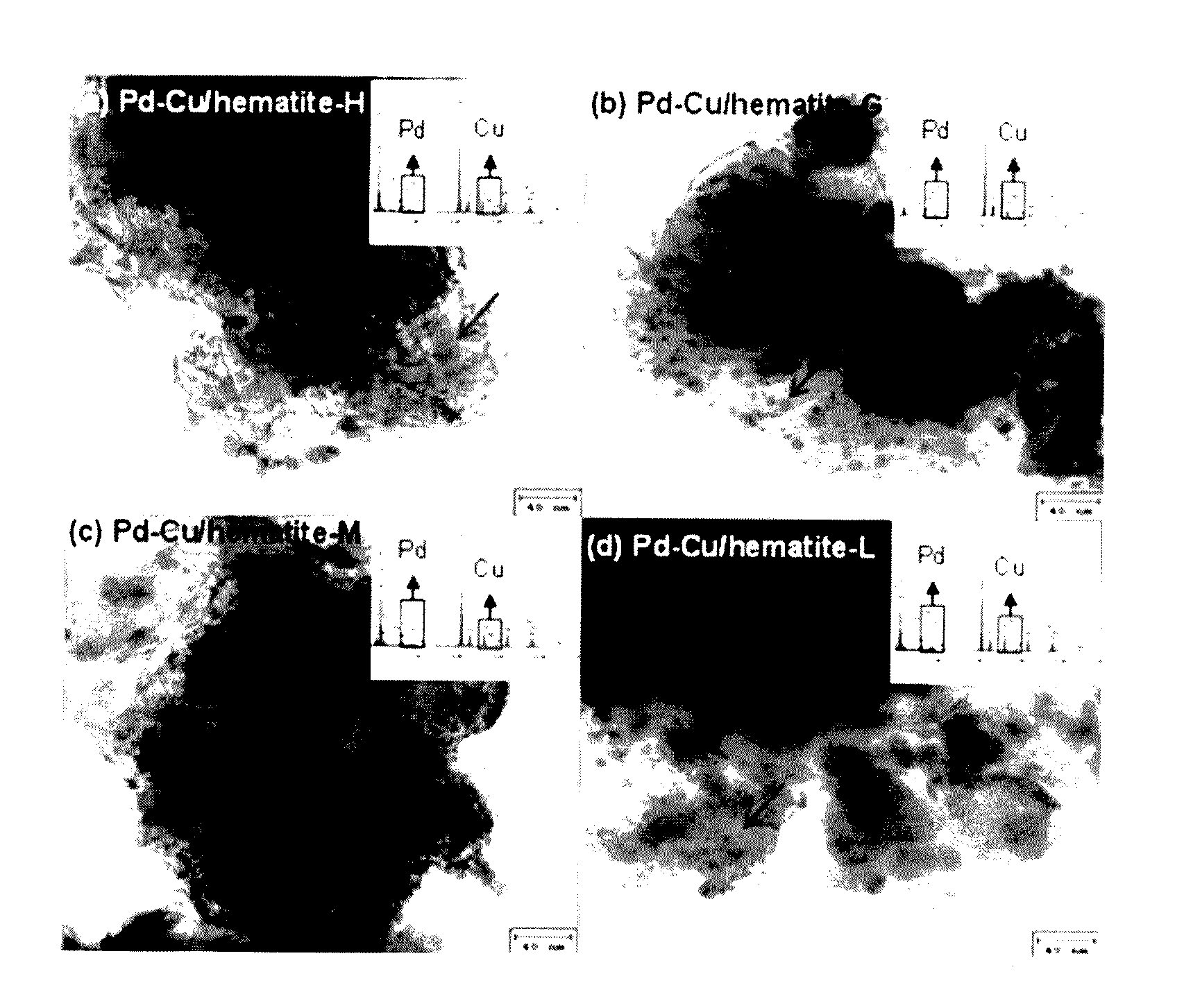
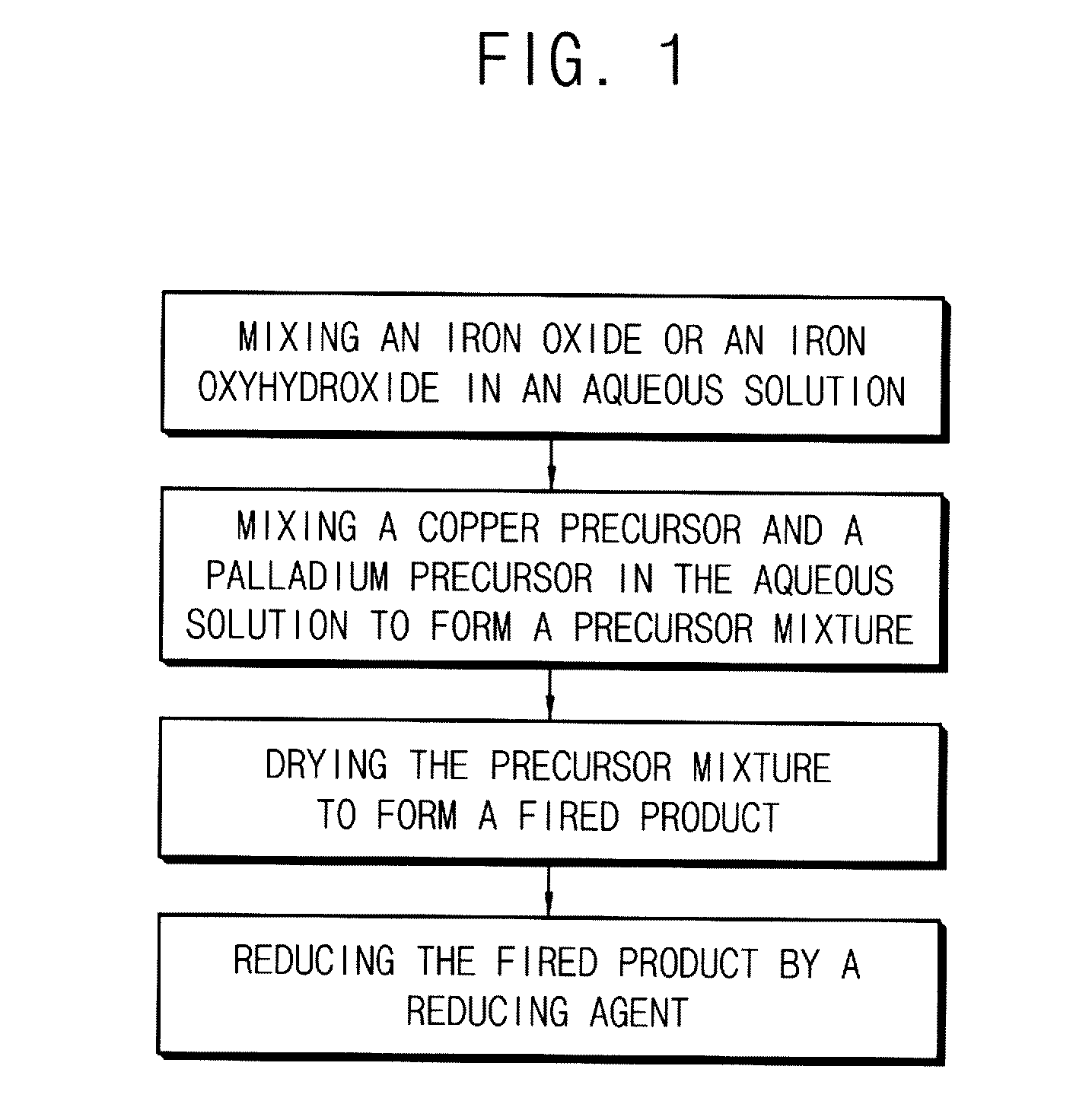
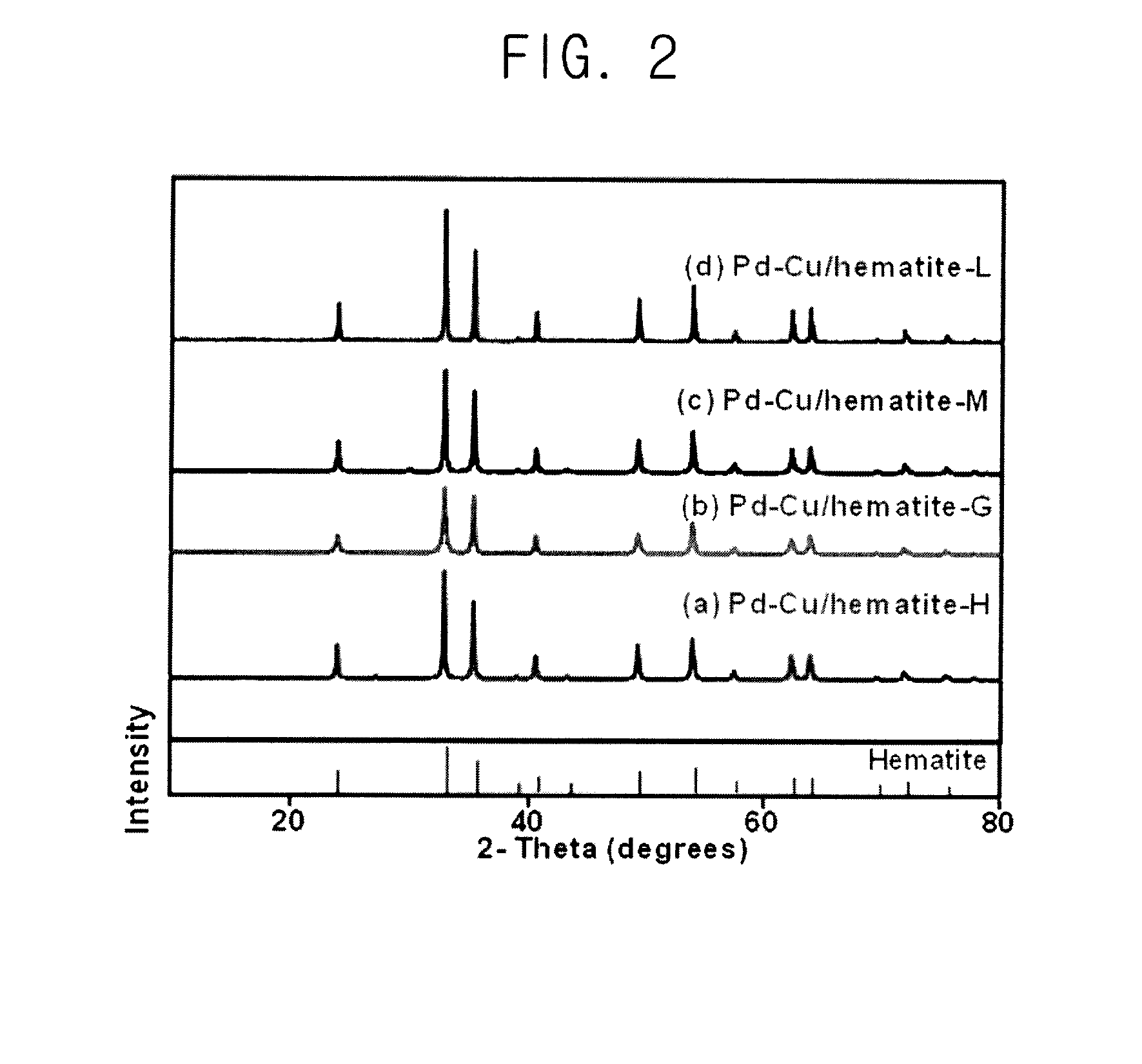
In a method of manufacturing a bimetallic catalyst for reductively decomposing nitrate nitrogen, a powder including a trivalent iron oxide, a powder including a trivalent iron oxyhydroxide powder or a combination thereof is mixed in an aqueous solution. A copper precursor and a palladium precursor are mixed in the aqueous solution to form a precursor mixture. The precursor mixture is dried. The dried precursor mixture is fired at a temperature from about 300° C. to about 450° C. to form a fired product. The fired product is reduced by a reducing agent. A hydrochloric acid solution is mixed in the aqueous solution, or mixed with the copper precursor or the palladium precursor.
Owner:KOREA ADVANCED INST OF SCI & TECH
Method for preparing sulfur trioxide by utilizing gypsum and equipment system thereof
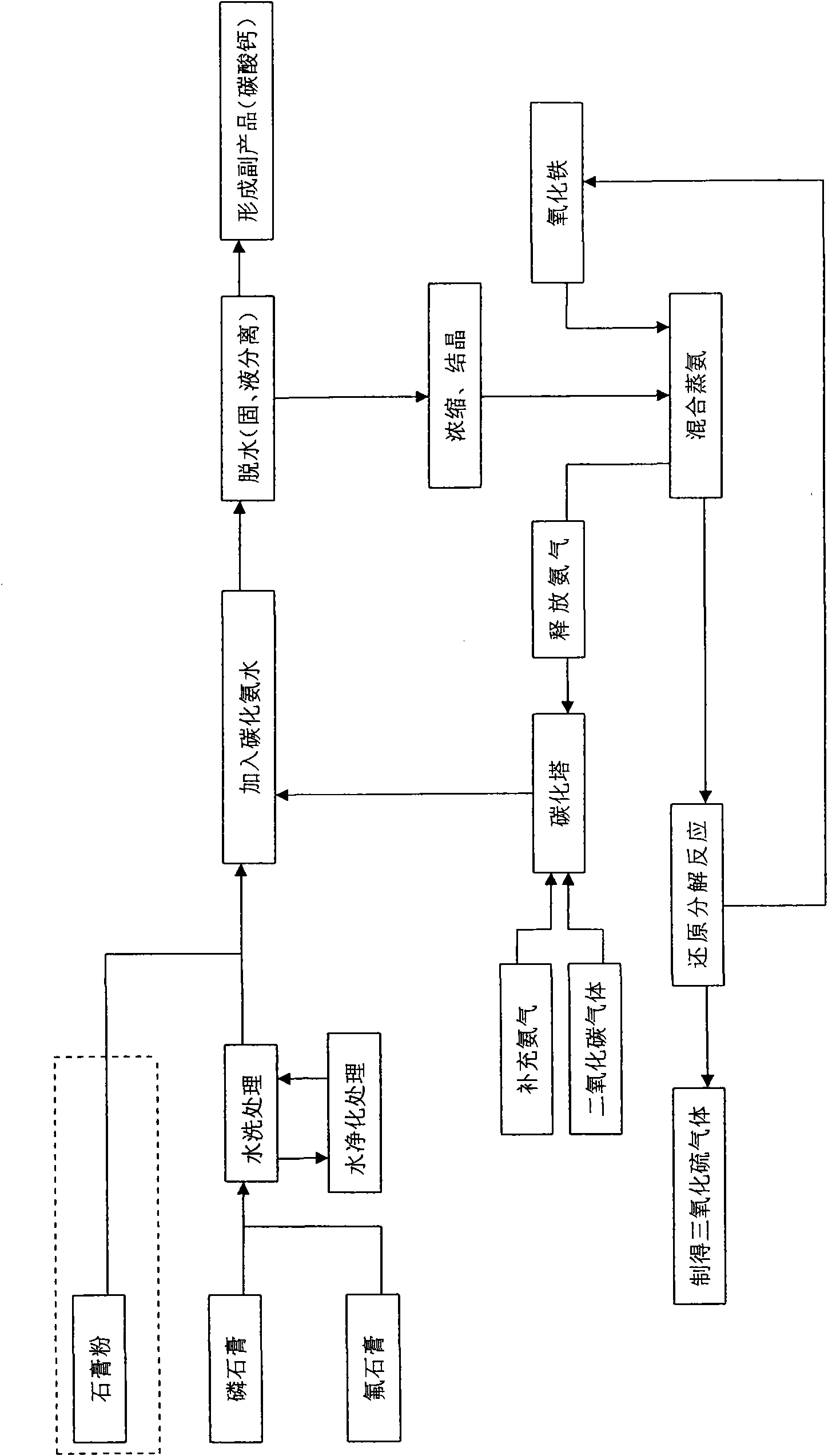
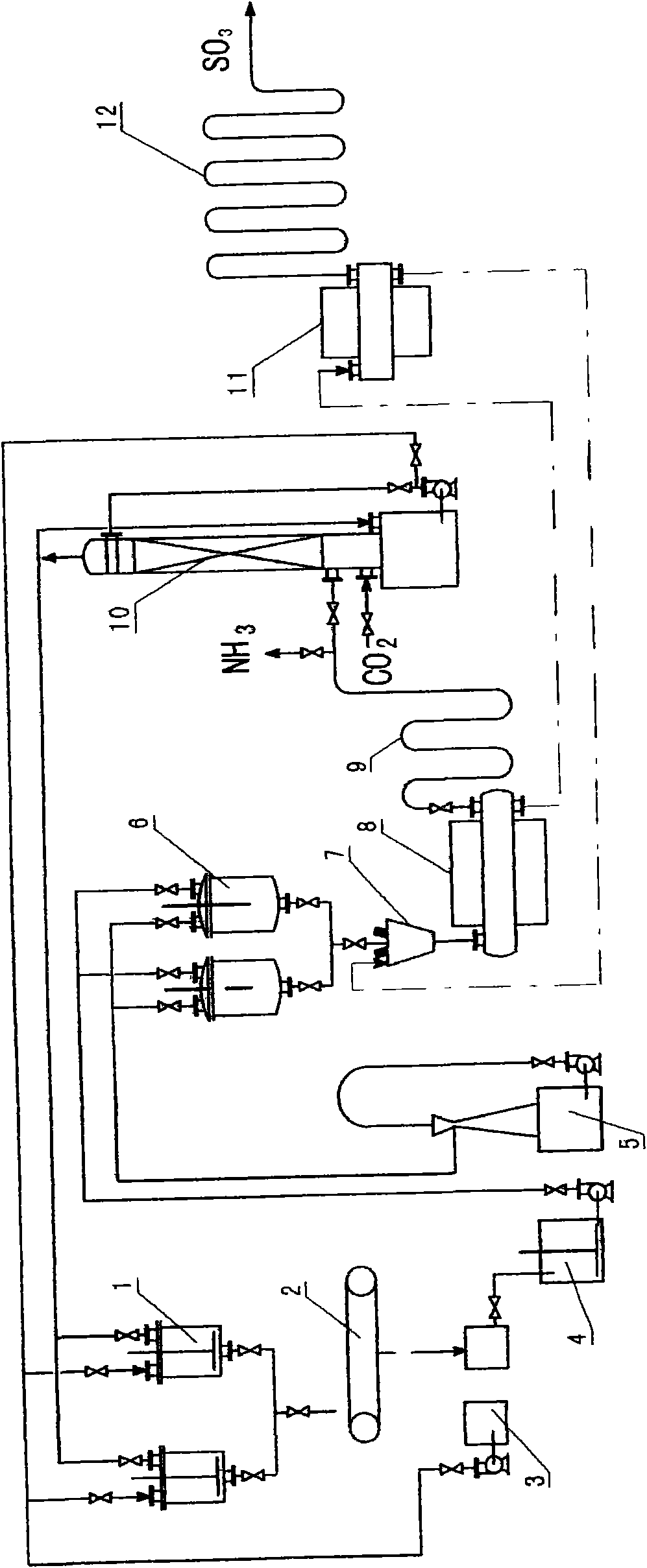
InactiveCN101554998AReduce manufacturing costSave resourcesSulfur-trioxide/sulfuric-acidReductive decompositionDecomposition
The invention relates to a method for preparing sulfur trioxide by utilizing gypsum and an equipment system thereof, aiming to provide a new technology which takes phosphogypsum or fluorine gypsum (also including ordinary gypsum) as raw materials to prepare the sulfur trioxide, and the matched equipment system thereof. The invention comprises the following steps: (1) double decomposition reaction: gypsum slurry is added into ammonium bicarbonate or carbonized ammonia water for reacting to obtain ammonium sulfate solution and calcium carbonate; then the steps of (2) dewater treatment, (3) concentration treatment, (4) mixing ammonia distillation treatment and (5) reductive decomposition reaction are sequentially carried out on the ammonium sulfate solution, and a sulfur trioxide gas product is obtained.
Owner:杭州卫士实业有限公司
Method for preparing calcium sulphide by decomposing phosphogypsum through hydrogen sulfide tail gas
InactiveCN105129742AReduce processingImprove reducibilityMagnesium/calcium/strontium/barium sulfides/polysulfidesReductive decompositionThiourea
The invention discloses a method for preparing calcium sulphide by decomposing phosphogypsum through hydrogen sulfide tail gas, and belongs to the field of phosphogypsum resource utilization. The phosphogypsum is dried and ground so that the phosphogypsum can be reduced and decomposed under Co and H2S tail gas to prepare CaS, decomposing temperature is 700-950 DEG C, the decomposing rate of the phosphogypsum is larger than and equal to 95%, and the productivity of calcium sulfide can reach over 85%. The production process is simple and easy to implement, the raw material is a large amount of the solid waste phosphogypsum, the prepared calcium sulfide can be used for preparing thiourea, sodium sulfide, sulphur, sulfuric acid and other chemical products and can also be used in the industries such as environmental protection and heavy metal treatment, and a new concept is provided for comprehensive utilization of the phosphogypsum.
Owner:KUNMING UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com