Sulfide solid electrolyte
A technology of solid electrolytes and sulfides, applied in solid electrolytes, sulfide conductors, non-aqueous electrolytes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] · Electrolyte synthesis
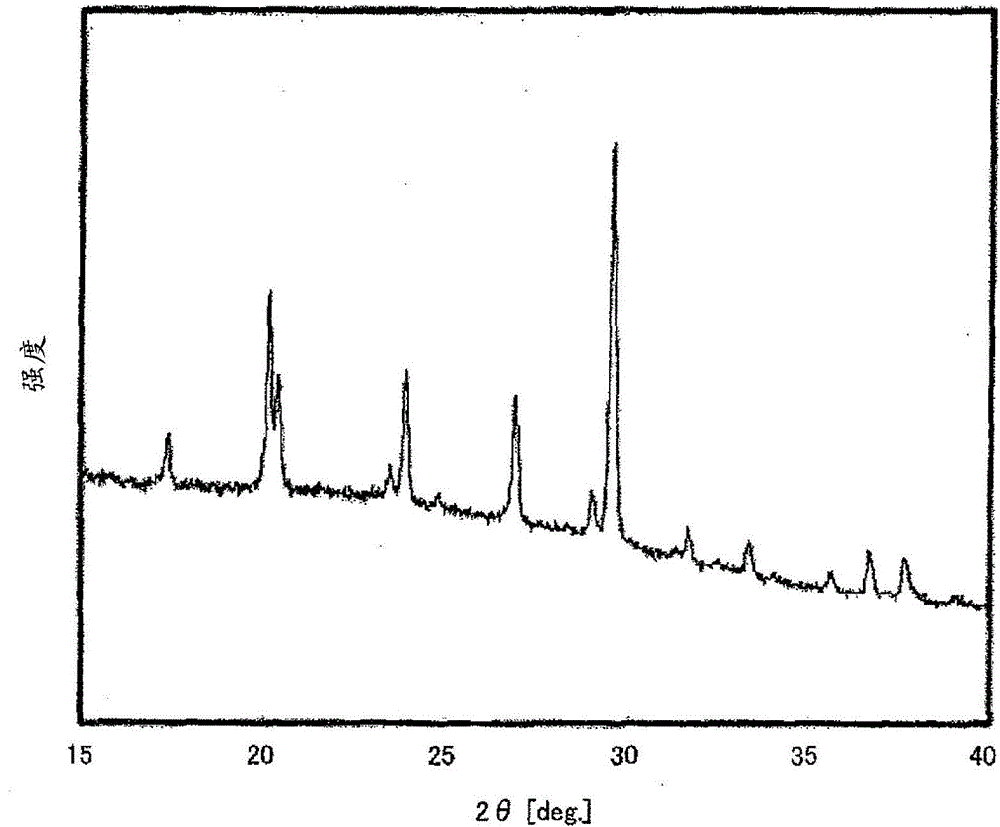
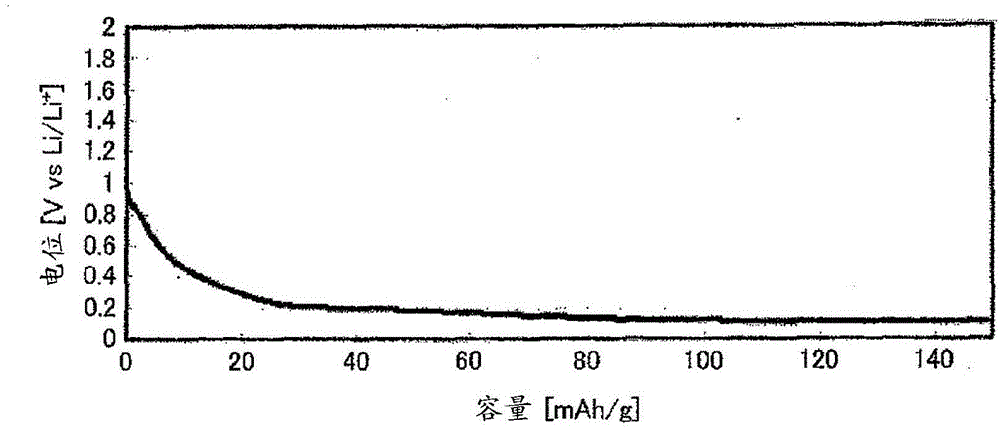
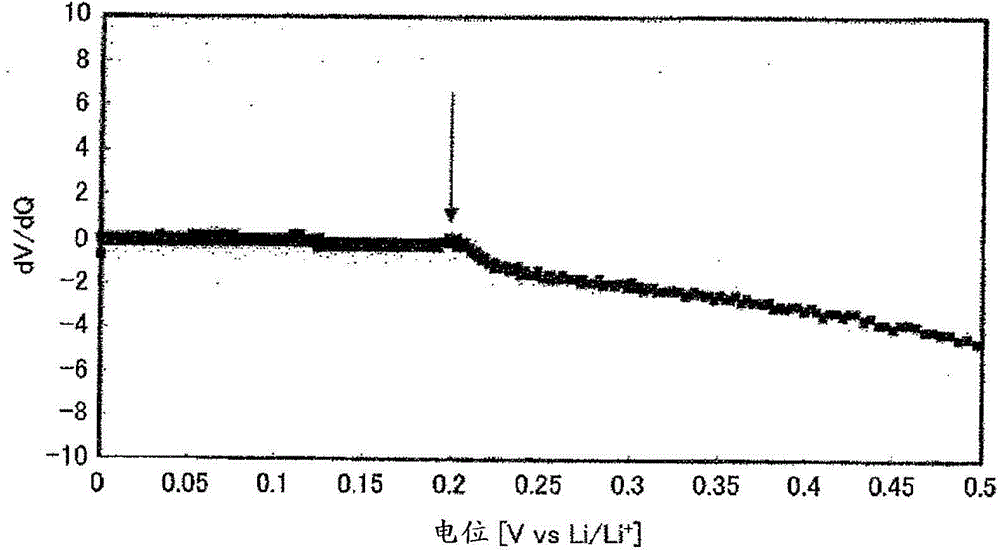
[0047] In an argon environment, weigh 0.425560g of Li 2 S (manufactured by Nippon Chemical Industry Co., Ltd.), 0.3796162 g of P 2 S 5 (manufactured by Aldrich), 0.125778 g of GeS 2 (manufactured by High Purity Chemical Research Institute Co., Ltd.) and 0.069045 g of Al 2 S 3 (manufactured by High Purity Chemical Laboratory Co., Ltd.), these were put into a zirconia bowl (capacity 45 ml) together with 10 zirconia balls with a diameter of 10 mm, and the bowl was sealed in an argon atmosphere. Then, this bowl was attached to a planetary ball mill (manufactured by FRITSCH, P-7), and it was made to rotate at 370 revolutions per minute, and was mixed for 40 hours. Next, the obtained mixed powder was put into a quartz tube, and the pressure in the quartz tube was reduced until the pressure in the quartz tube reached 30 Pa, and then sealed. Thereafter, the sealed quartz tube was heated at 550° C. for 8 hours, whereby the sulfide solid electrolyt...
Embodiment 2
[0054] The starting material when making the synthesized electrolyte is 0.397341g of Li 2 S (manufactured by Nippon Chemical Industry Co., Ltd.), 0.369102 g of P 2 S 5 (manufactured by Aldrich), 0.220129 g of GeS 2 (manufactured by High Purity Chemical Research Institute Co., Ltd.) and 0.013426g of Al 2 S 3 (manufactured by High Purity Chemical Laboratory Co., Ltd.), and the sulfide solid electrolyte according to Example 2 was synthesized in the same manner as in Example 1 except the above.
[0055] The composition of the sulfide solid electrolyte involved in the synthetic embodiment 2 is Li 3.385 Al 0.035 Ge 0.315 P 0.65 S 4 , the sulfide solid electrolyte involved in Example 2 is M0≈0.05385.
[0056] In addition, X-ray diffraction measurement was performed on the sulfide solid electrolyte related to Example 2 by the same method as in Example 1. show the result in Figure 4 . if will Figure 4 and figure 1 For comparison, they have peaks at the same position. T...
Embodiment 3
[0059] The starting material when making the synthesized electrolyte is 0.403205g of Li 2 S (manufactured by Nippon Chemical Industry Co., Ltd.), 0.414400 g of P 2 S 5 (manufactured by Aldrich), 0.129300 g of SnS 2 (manufactured by High Purity Chemical Research Institute Co., Ltd.) and 0.053094g of Al 2 S 3 (manufactured by High Purity Chemical Laboratory Co., Ltd.), and the sulfide solid electrolyte according to Example 3 was synthesized in the same manner as in Example 1 except for this.
[0060] The composition of the sulfide solid electrolyte involved in the synthetic embodiment 3 is Li 3.4125 Al 0.1375 sn 0.1375 P 0.725 S 4 , the sulfide solid electrolyte involved in Example 3 is M0≈0.18966.
[0061] In addition, X-ray diffraction measurement was performed on the sulfide solid electrolyte related to Example 3 by the same method as in Example 1. show the result in Figure 7 . if will Figure 7 and figure 1 For comparison, they have peaks at the same position....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com