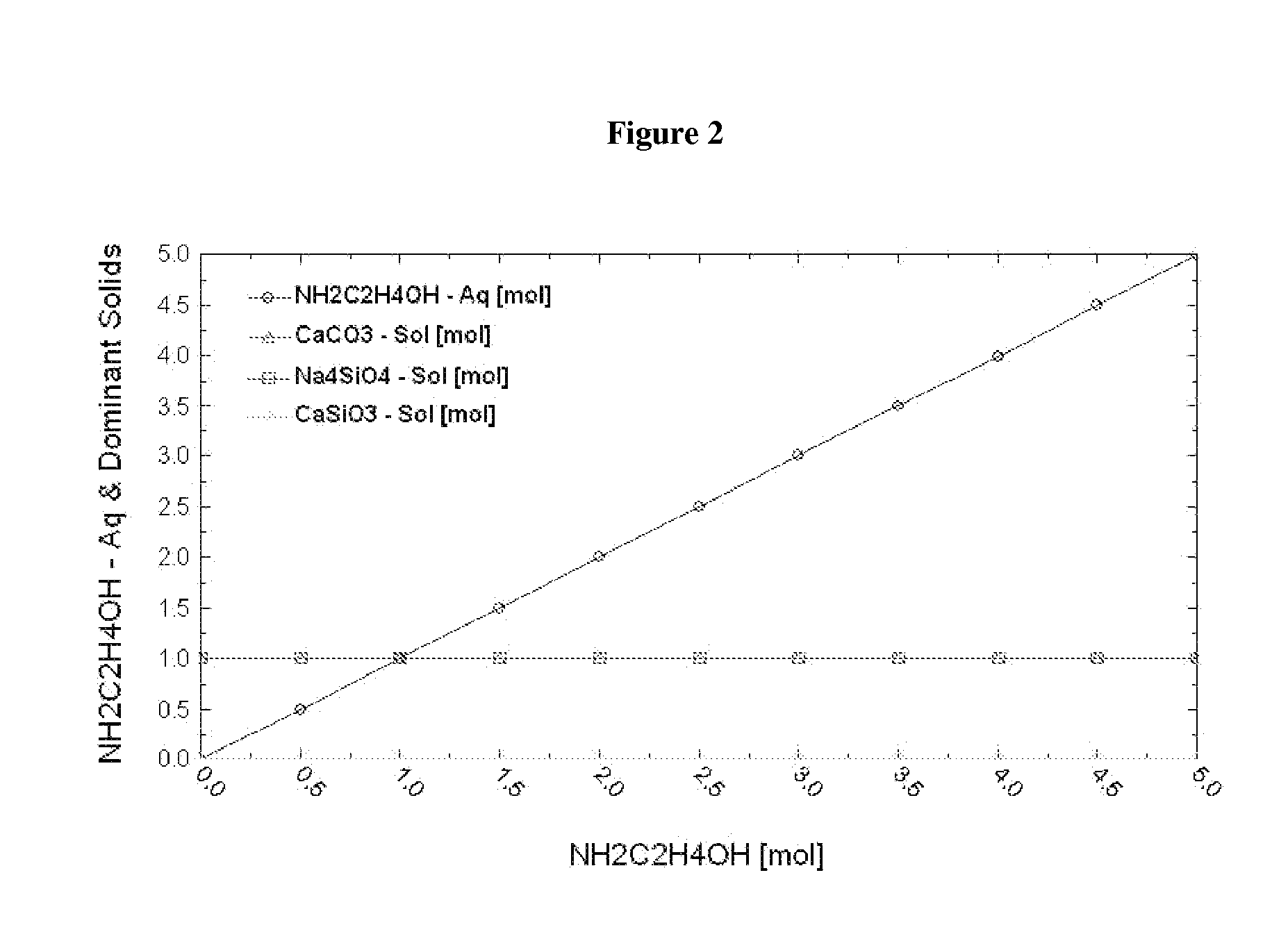
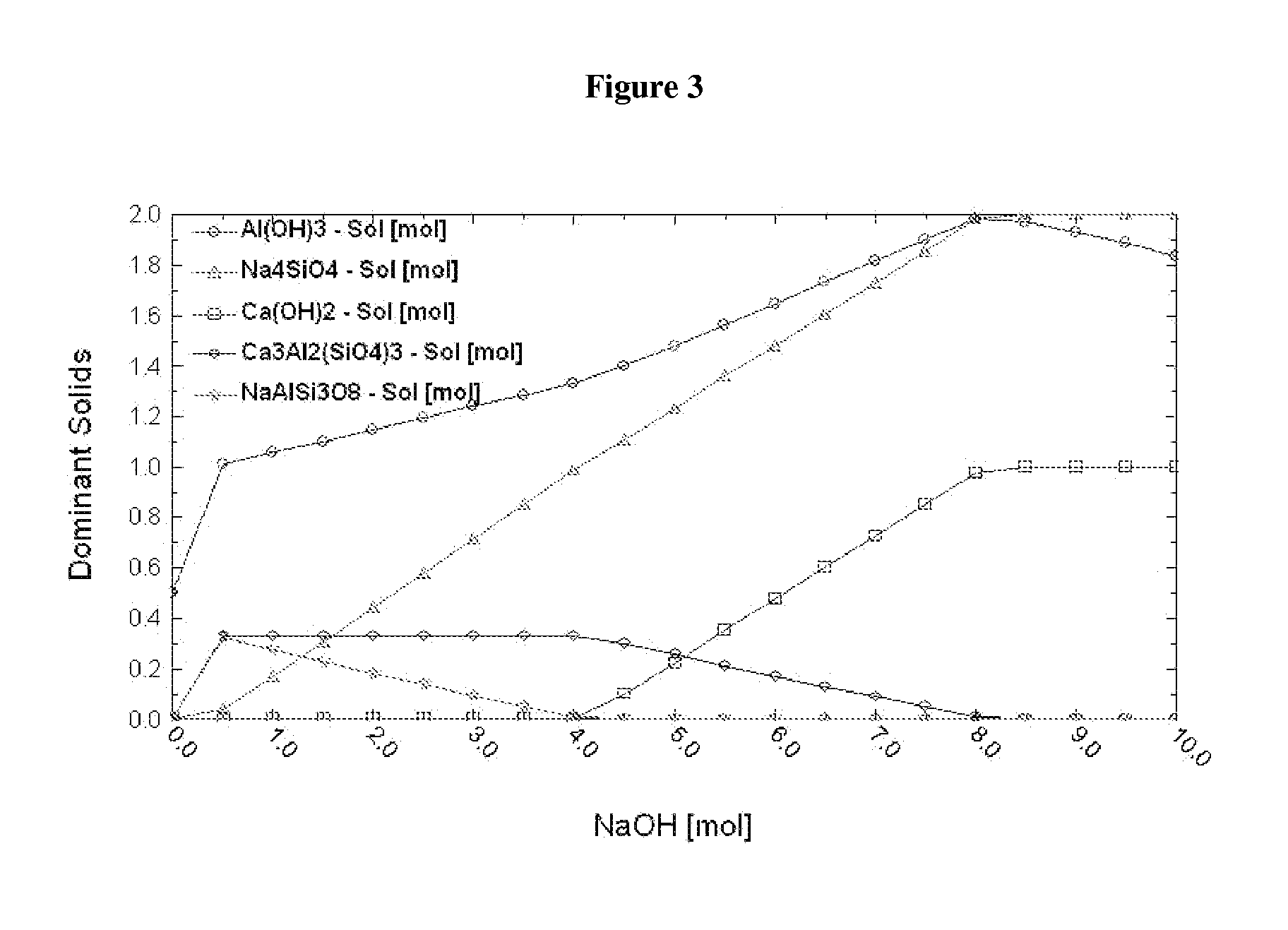
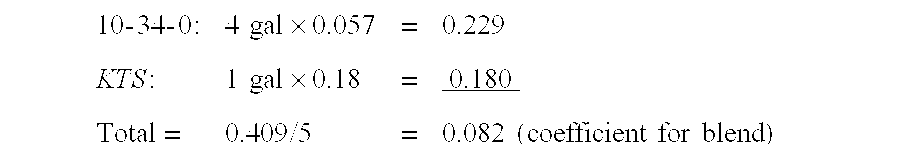Patents
Literature
149results about "Magnesium/calcium/strontium/barium sulfides/polysulfides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reactive formulations for a neutralization of toxic industrial chemicals
InactiveUS7125497B1Efficiently neutralizedHydrogen peroxideLiquid degasificationBoron trichlorideMalathion
Decontamination formulations for neutralization of toxic industrial chemicals, and methods of making and using same. The formulations are effective for neutralizing malathion, hydrogen cyanide, sodium cyanide, butyl isocyanate, carbon disulfide, phosgene gas, capsaicin in commercial pepper spray, chlorine gas, anhydrous ammonia gas; and may be effective at neutralizing hydrogen sulfide, sulfur dioxide, formaldehyde, ethylene oxide, methyl bromide, boron trichloride, fluorine, tetraethyl pyrophosphate, phosphorous trichloride, arsine, and tungsten hexafluoride.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
Sequestration of a gas emitted by an industrial plant
InactiveUS20110182799A1Eliminate needCalcium/strontium/barium carbonatesAmmonium nitratesNitrogenNitrogen gas
A method of sequestering a multi-element gas emitted by an industrial plant is described herein, the method comprising: contacting a solution, including a first reactant comprising a multi-element gas emitted by an industrial plant and at least one gas absorber comprising nitrogen, for example ammonia or an amine, with a solid, including a second reactant, under conditions that promote a reaction between the first reactant and the second reactant to provide a first product, which incorporates one or more elements of the multi-element gas, thereby sequestering the multi-element gas.
Owner:RUTGERS THE STATE UNIV
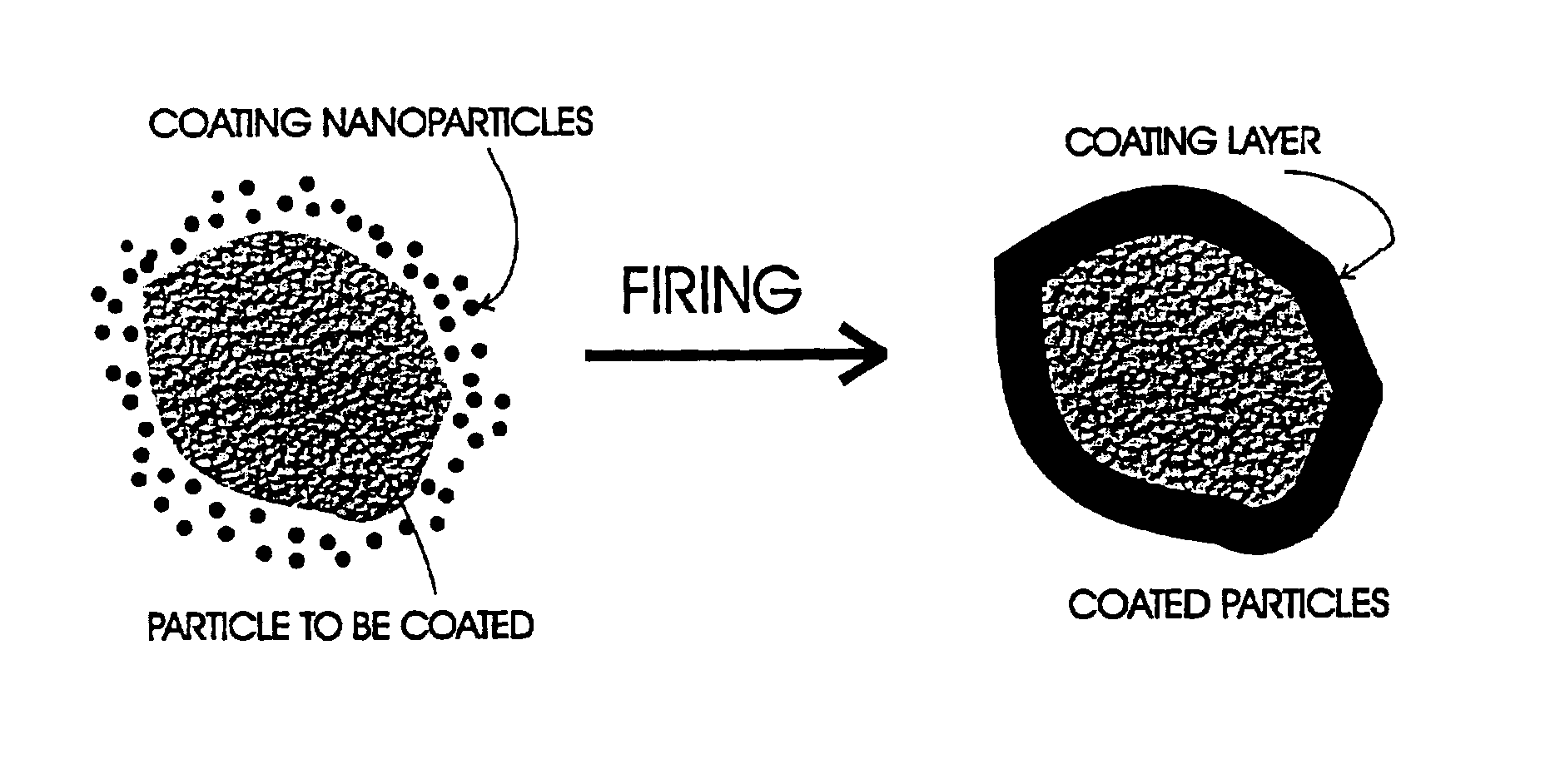
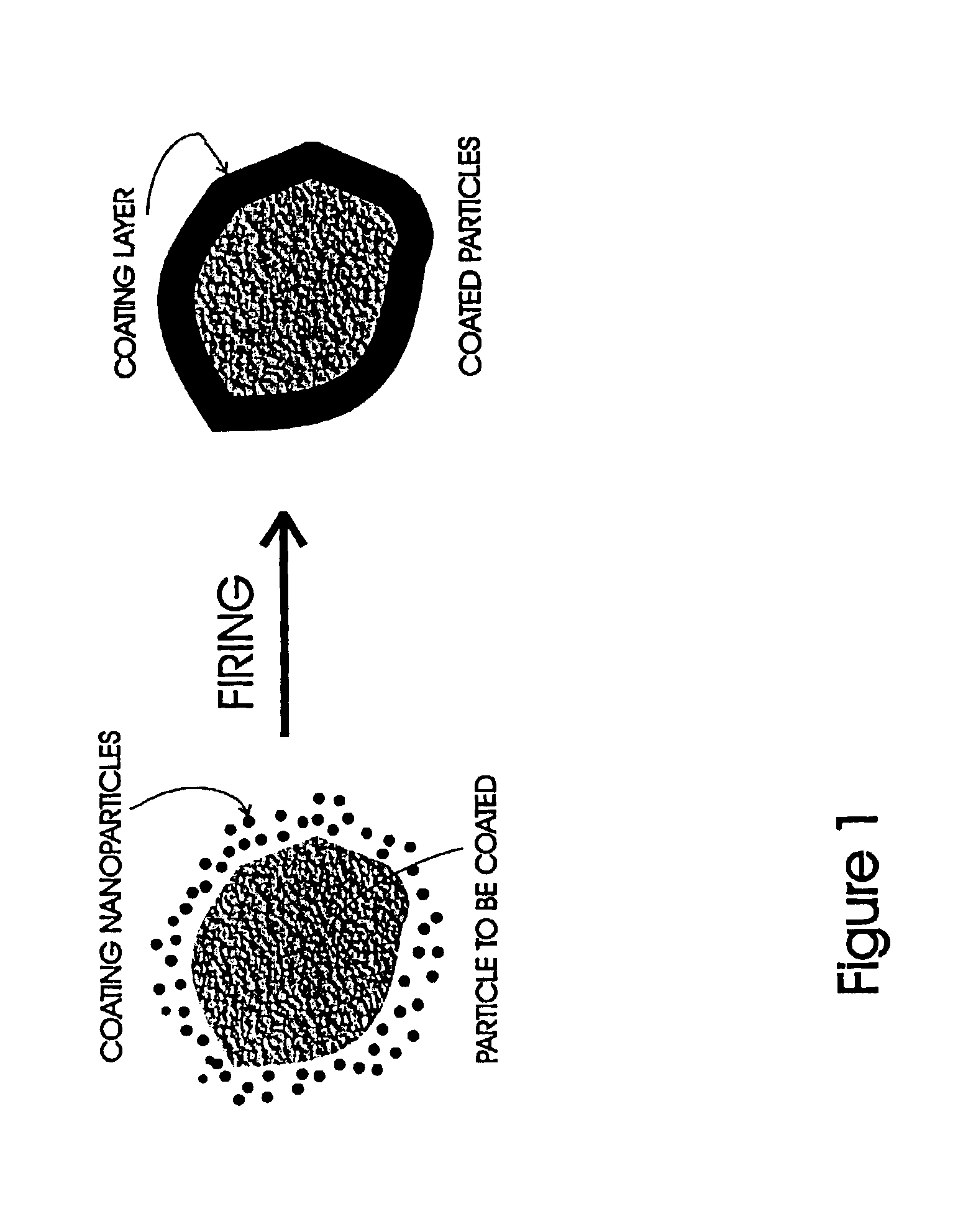
Method of coating micrometer sized inorganic particles
A method for coating moisture sensitive inorganic particles, such as phosphors, comprising mixing the inorganic particles having a particle size of about 1 to 100 micrometers with nanometer-sized coating particles, and firing the mixture to soften or melt the nanometer-sized particles about the inorganic particles, forming a moisture impervious coating. The coated particles are washed to remove excess particles, and dried. Coated moisture sensitive phosphor particles are impervious to moisture.
Owner:SARNOFF CORP
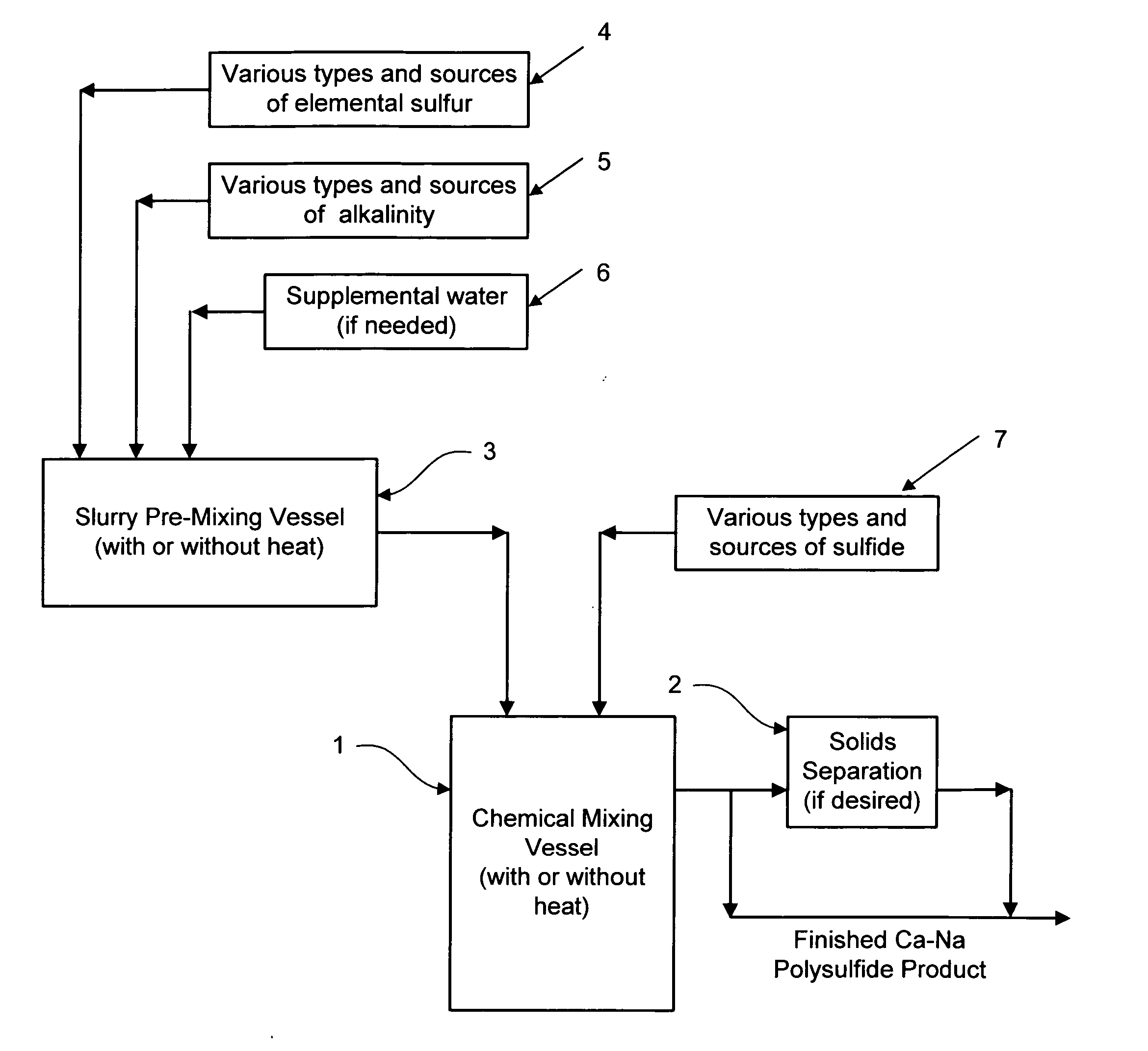
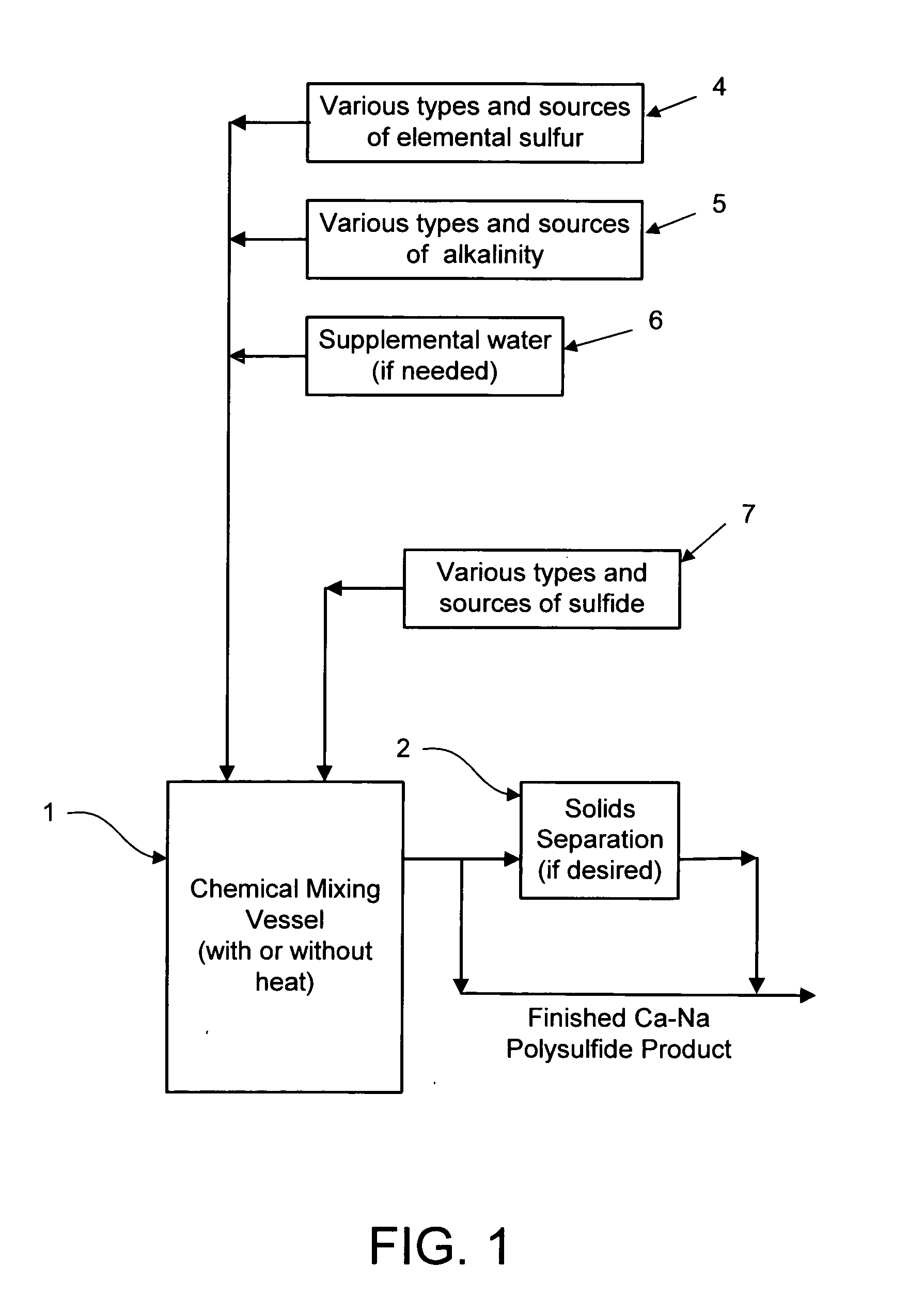
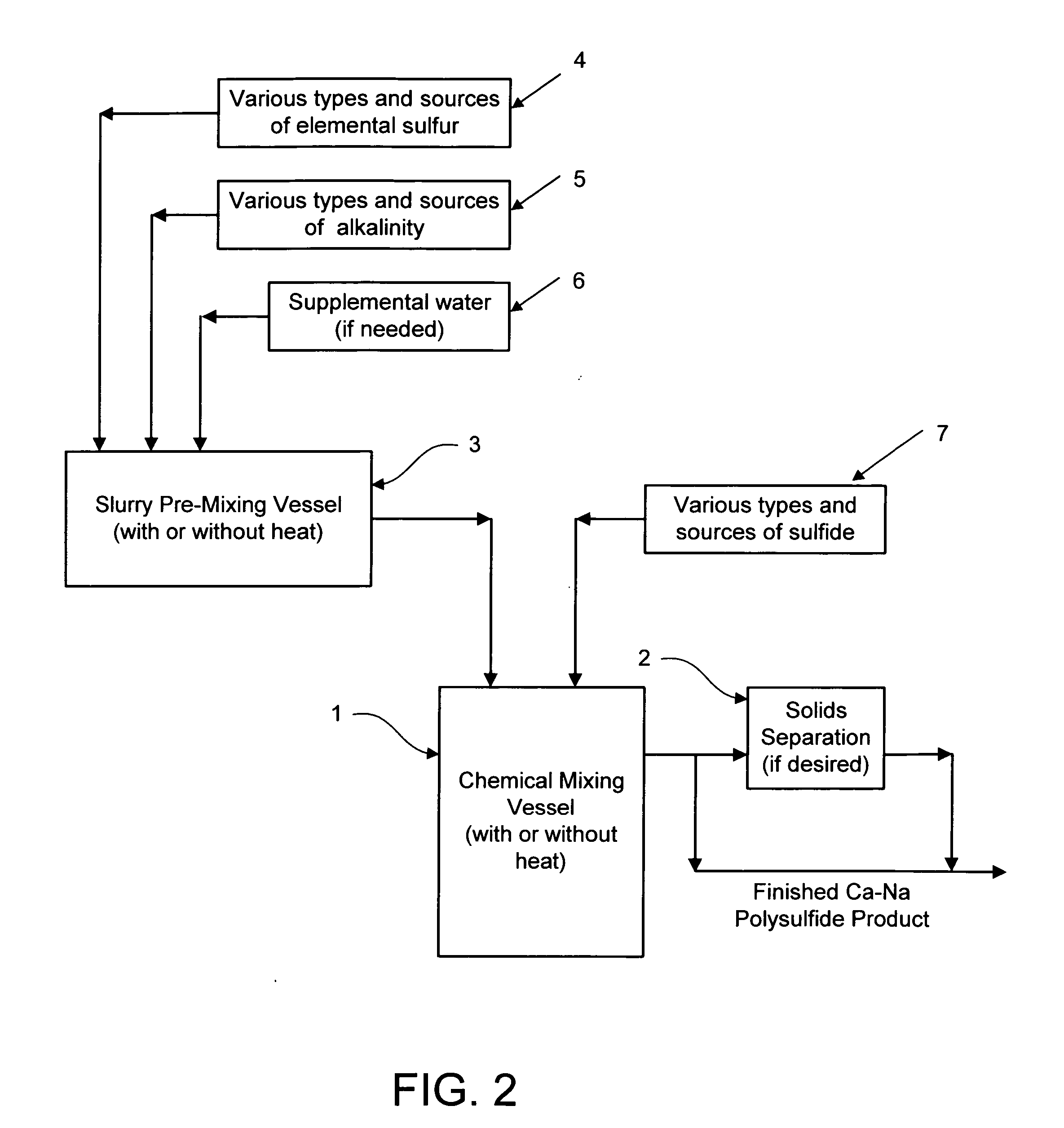
Calcium-sodium polysulfide chemical reagent and production methods
Owner:REDOX TECH GRP
Scavenger for aldehyde(s) and a manufacturing method of a woody panel using the same
InactiveUS20090130474A1Ease of evaluationImprove trapping efficiencyOrganic chemistrySulfate/bisulfate preparationScavengerRoom temperature
Regarding the scavenger for aldehyde(s) used at the time of manufacturing a woody panel using woody materials and formaldehyde-based binders, the scavenger for aldehyde(s) without lowering of trapping properly even when a surface of said woody panel is sanded and having an excellent trapping property of trapping formaldehyde is provided. Further, the method of manufacturing a woody panel using a scavenger for aldehyde(s) and a woody panel are provided.At least one kind of compound for trapping aldehyde(s) being solid at a room temperature is included and said compound for trapping aldehyde(s) is defined to be a powdery scavenger for aldehyde(s) having a property of generating acidic gas, in particular, sulfurous acid gas by heating and said compound for trapping aldehyde(s) is added to a binder or woody materials followed by a hot press, thereby manufacturing a woody panel.
Owner:IPPOSHA OIL INDS
Sorbents for Removal of Mercury From Flue Gas Cross Reference To Related Applications
Systems are disclosed for making and using micro-porous particulates at least partially composed of metal sulfides, particularly alkaline earth metal and transition metal sulfides, as sorbents for removal of mercury from flue gas. Calcium sulfide micro-porous powders derived from the high temperature reduction of calcium sulfate and calcium sulfite are disclosed to be reactive substrates for a group of sorbents for adsorption of mercury from coal combustion flue gases produced by the utilities industry, as well as from natural gas and gaseous and liquid hydrocarbons. The sorbents are useful for cost-effectively adsorbing elemental mercury and oxidized mercury species such as mercuric chloride from flue gases, including those containing acid gases (e.g., SO.sub.2, NO and NO.sub.2, and HCl), over a wide range of temperatures.
Owner:CHEM PROD CORP
Preparation for metal sulfide
InactiveCN1613750ALow costEasy to getAlkali metal sulfides/polysulfidesSulfide/polysulfide preparationOrganic solventMetallic sulfide
Owner:WUHAN UNIV
Catalyst for reducing mercury, a mercury conversion unit, and an apparatus for measuring total mercury in combustion exhaust gas by using the same
InactiveUS20070232488A1Reduce functionImprove accuracyCalcium/strontium/barium carbonatesUsing liquid separation agentCombustionPhosphate
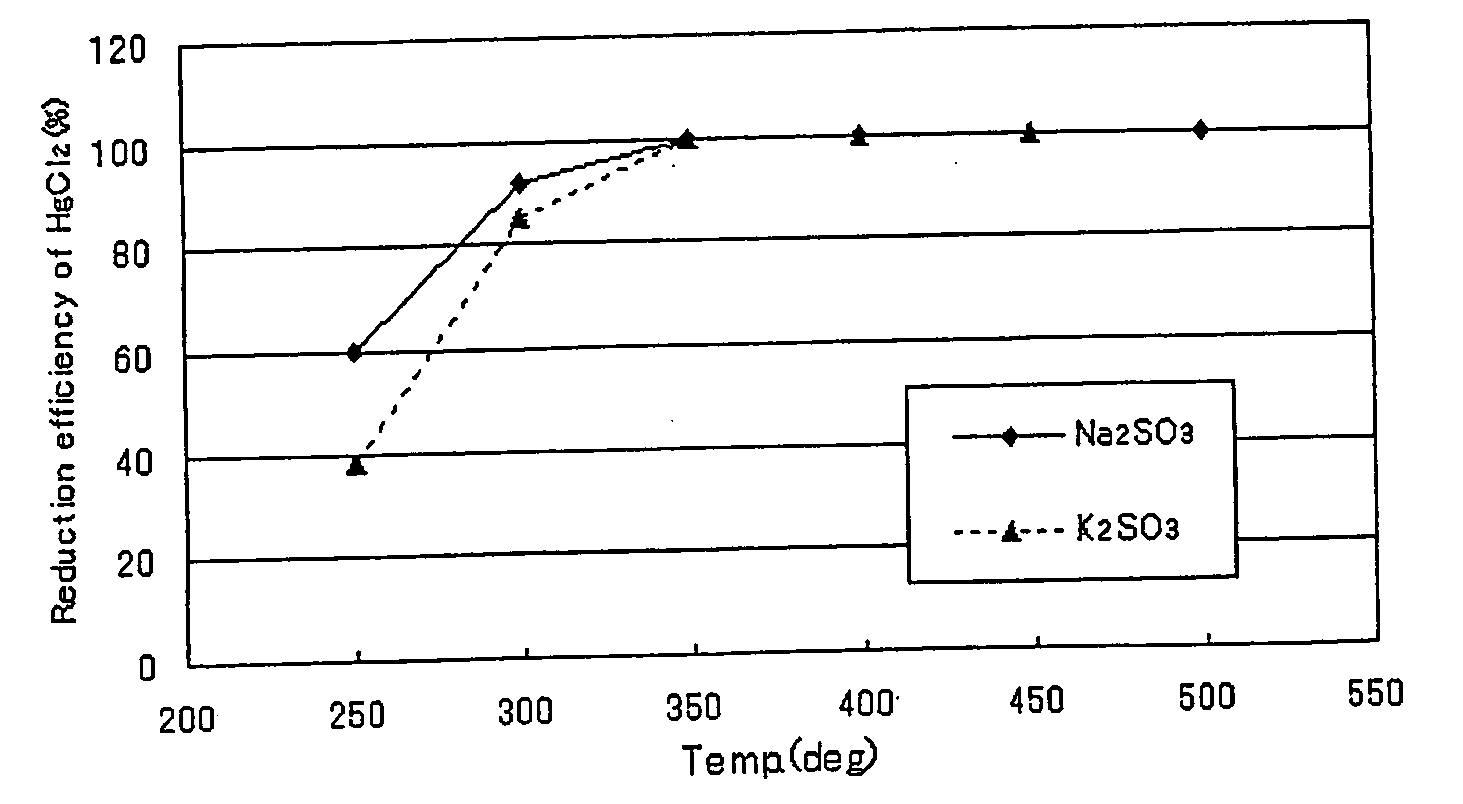
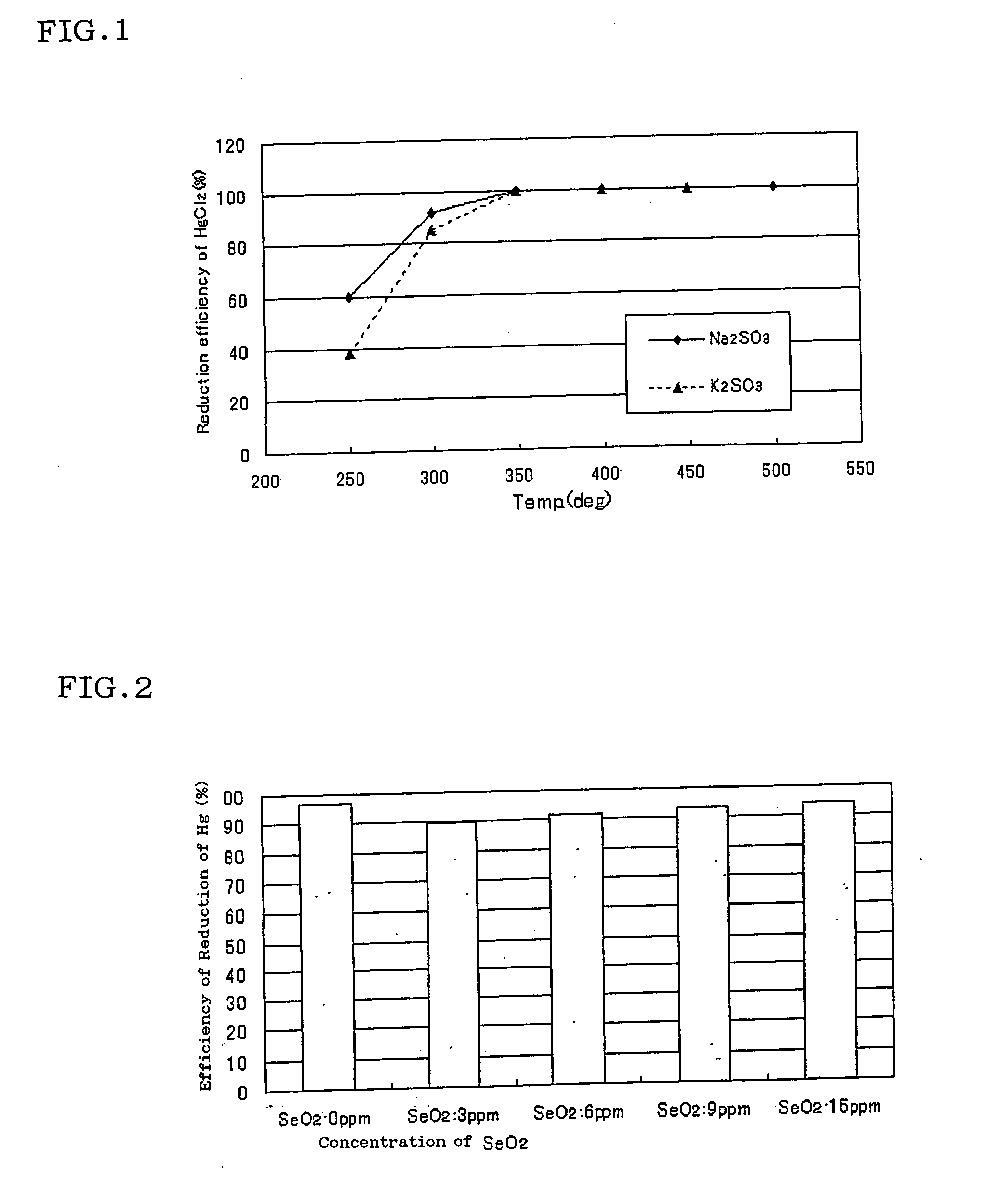
The present invention relates to a catalyst for reducing mercury, which comprises a reagent comprising any of the sulfites of potassium, sodium, calcium and magnesium, or any of the phosphates thereof, or a combination of them, as a main reagent of a catalyst component. And the present invention relates to the catalyst for reducing mercury, wherein the catalyst component is mixed with a different salt as an agent for inhibiting crystallization of the catalyst component.
Owner:HORIBA LTD
Aqueous catalyst sulfiding process
InactiveUS20120322653A1Pulp liquor regenerationCatalyst activation/preparationMetalInorganic chemistry
Owner:SHELL OIL CO
Desulfuration and mercury-removing combined smoke purifying process and system based on magnesium oxide method desulfuration process
InactiveCN102205203AWith resource utilizationRealize resource utilizationDispersed particle separationMagnesium/calcium/strontium/barium sulfides/polysulfidesGas phaseSulfide
The invention discloses a desulfuration and mercury-removing combined smoke purifying process and system based on a magnesium oxide method desulfuration process. The desulfuration and mercury-removing combined smoke purifying process comprises the steps of: improving a pre-washing tower in the magnesium oxide method desulfuration process, circularly spraying original smoke by adopting a halogen oxidant solution to realize full oxidization of elementary substance mercury Hg0 in smoke; transforming oxidized gas-phase bivalent mercury ions into liquid-phase bivalent mercury ions through an improved wet-type magnesium oxide method desulfuration process; and transforming the liquid-phase bivalent mercury ions in a wastewater treatment system into a stable mercuric sulfide precipitate through adding a mercury stabilizing agent. The system mainly comprises the pre-washing tower, a desulfuration tower, a cyclone separator, a vacuum belt fulter, a wastewater basin and a sedimentation basin, wherein the pre-washing tower is used for preparing a mercury oxidant and a magnesium oxide solution and oxidizing mercury; the desulfuration tower is used for carrying out desulfuration and mercury removal on smoke; the cyclone separator and the vacuum belt fulter are used for treating side products; and the wastewater basin and the sedimentation basin are respectively used for treating wastewater and the mercuric sulfide precipitate. The process can be used for effectively removing divalent mercury and elementary substance mercury in the smoke on the premise of ensuring the advantages of the magnesium oxide method desulfuration process, and is suitable for reconstructing the traditional magnesium oxide method desulfuration process.
Owner:CECEP L&T ENVIRONMENTAL TECH
Sorbents for removal of mercury from flue gas cross reference to related applications
InactiveUS7771700B2Solid waste managementUsing liquid separation agentParticulatesAlkaline earth metal
Owner:CHEM PROD CORP
Process for preparing calcium thiosulfate solution
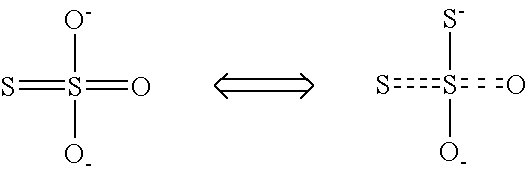
InactiveUS6984368B2Thiosulfates/dithionites/polythionitesMagnesium/calcium/strontium/barium sulfides/polysulfidesParticulatesSulfur
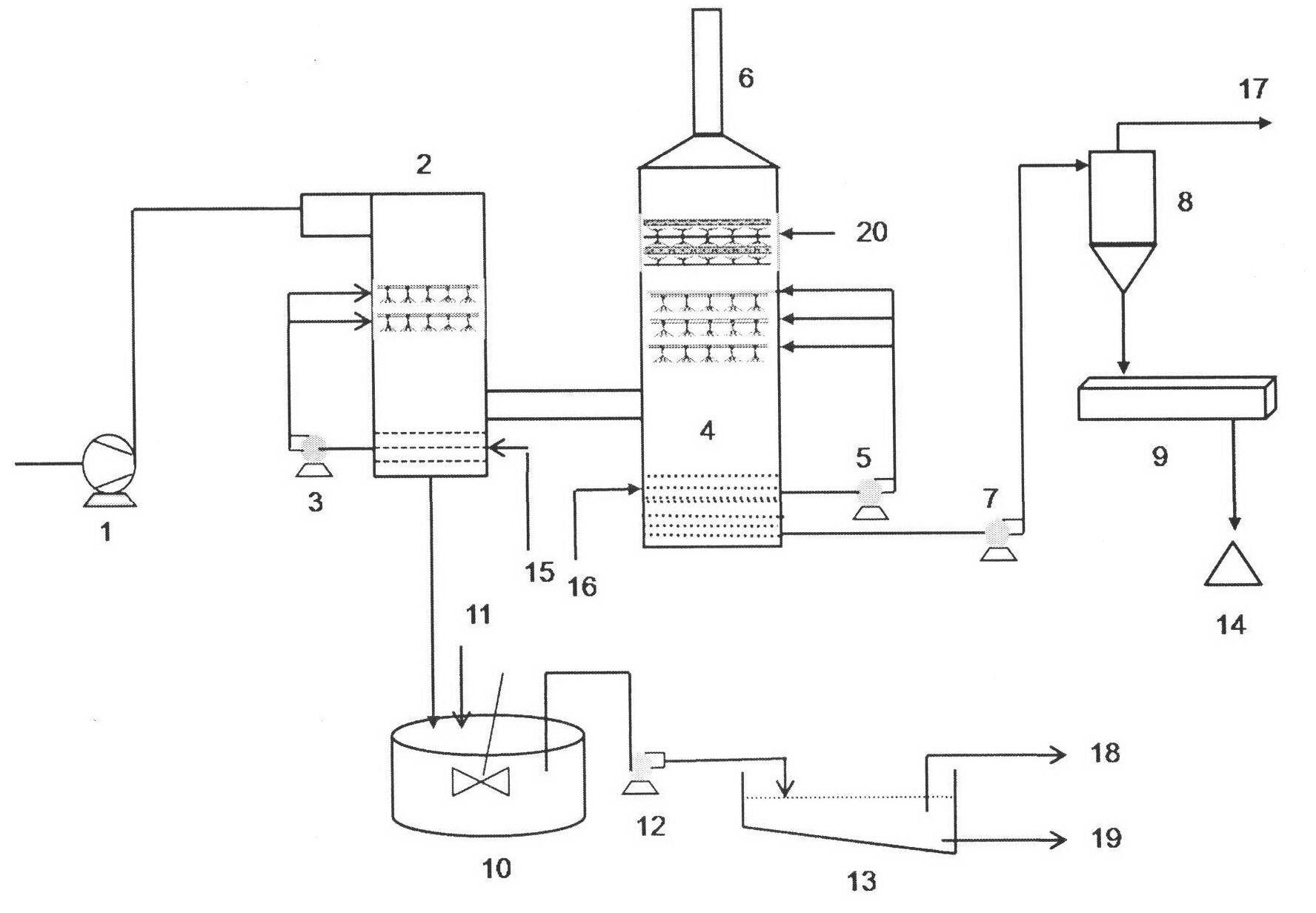
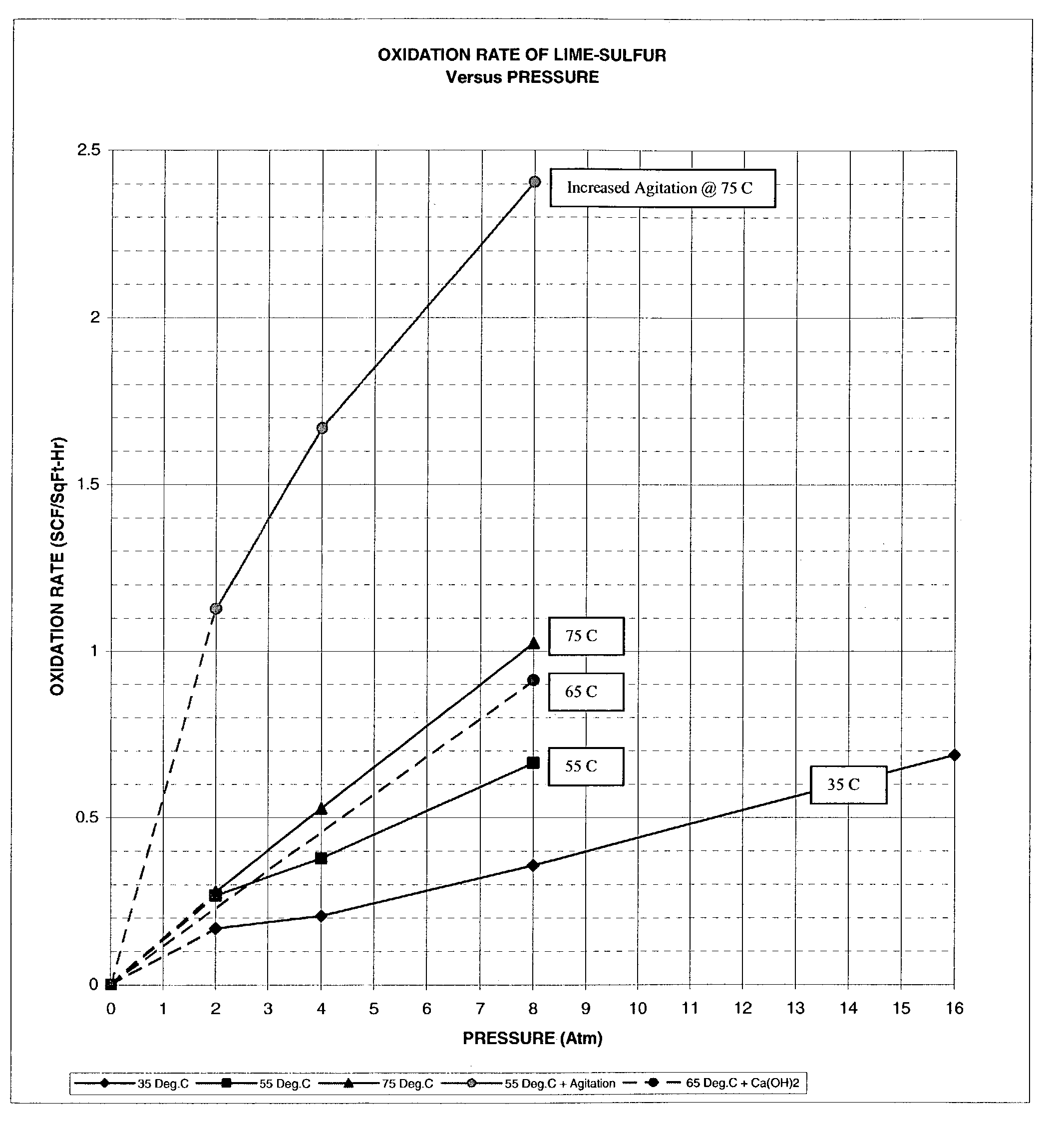
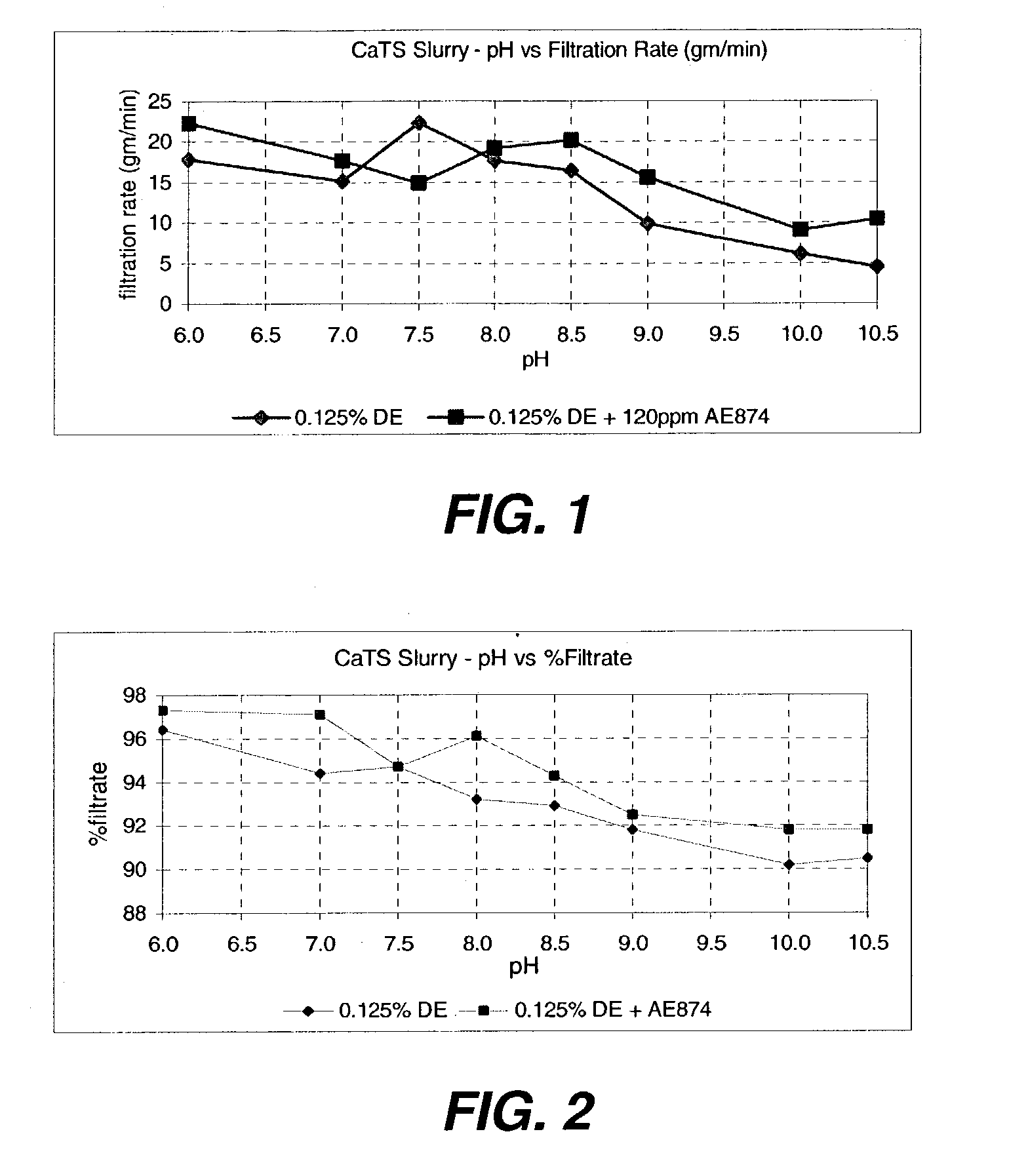
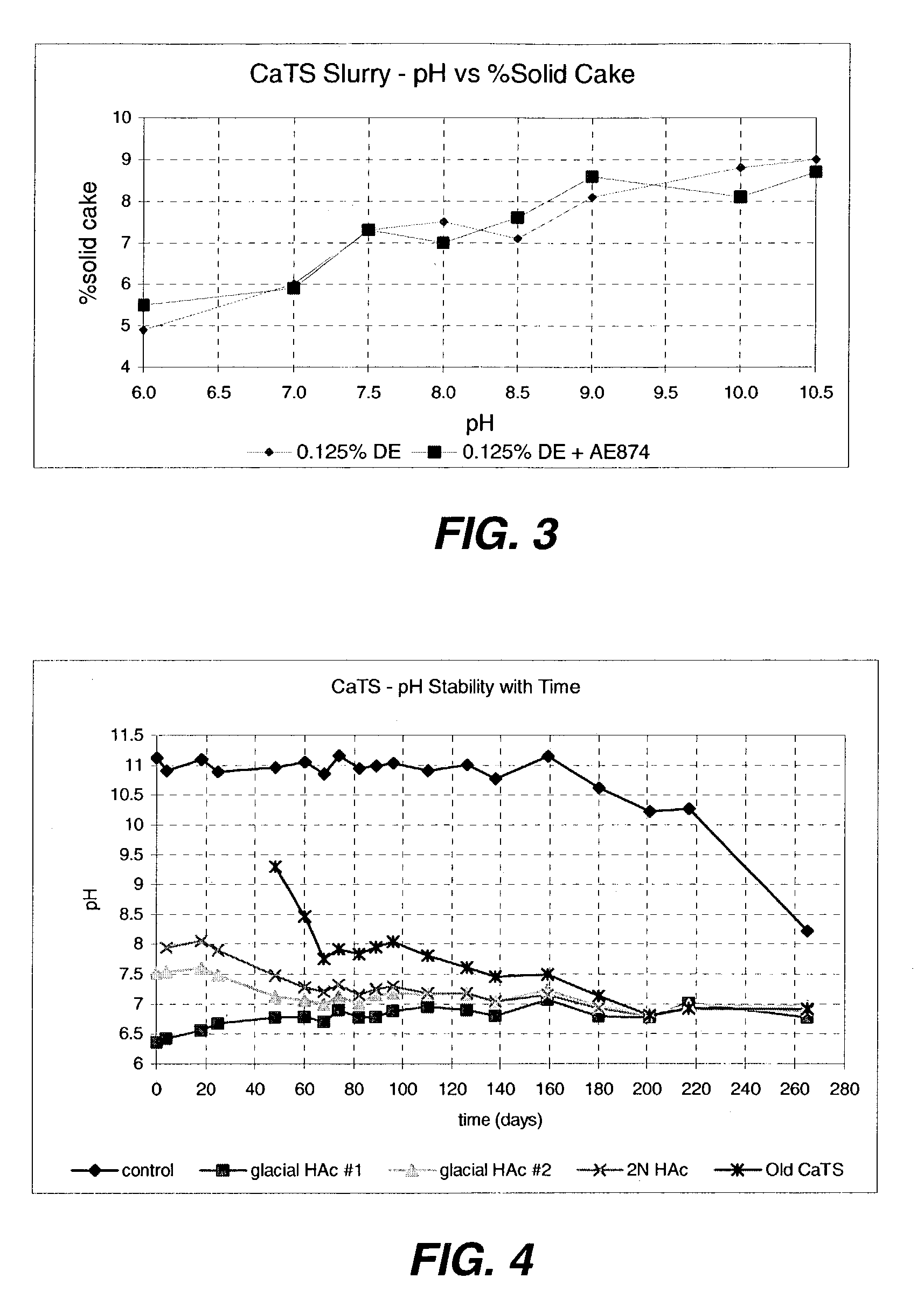
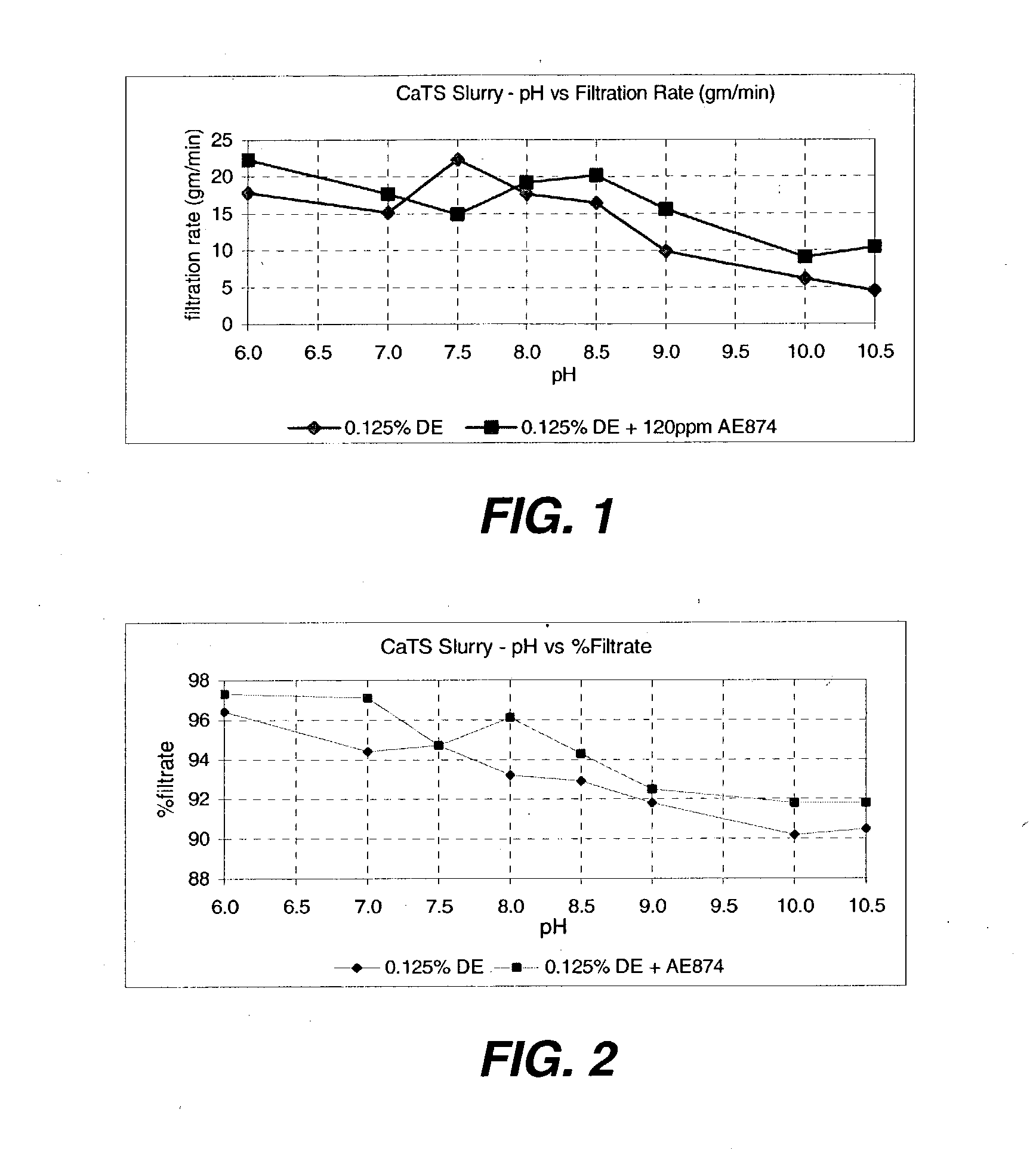
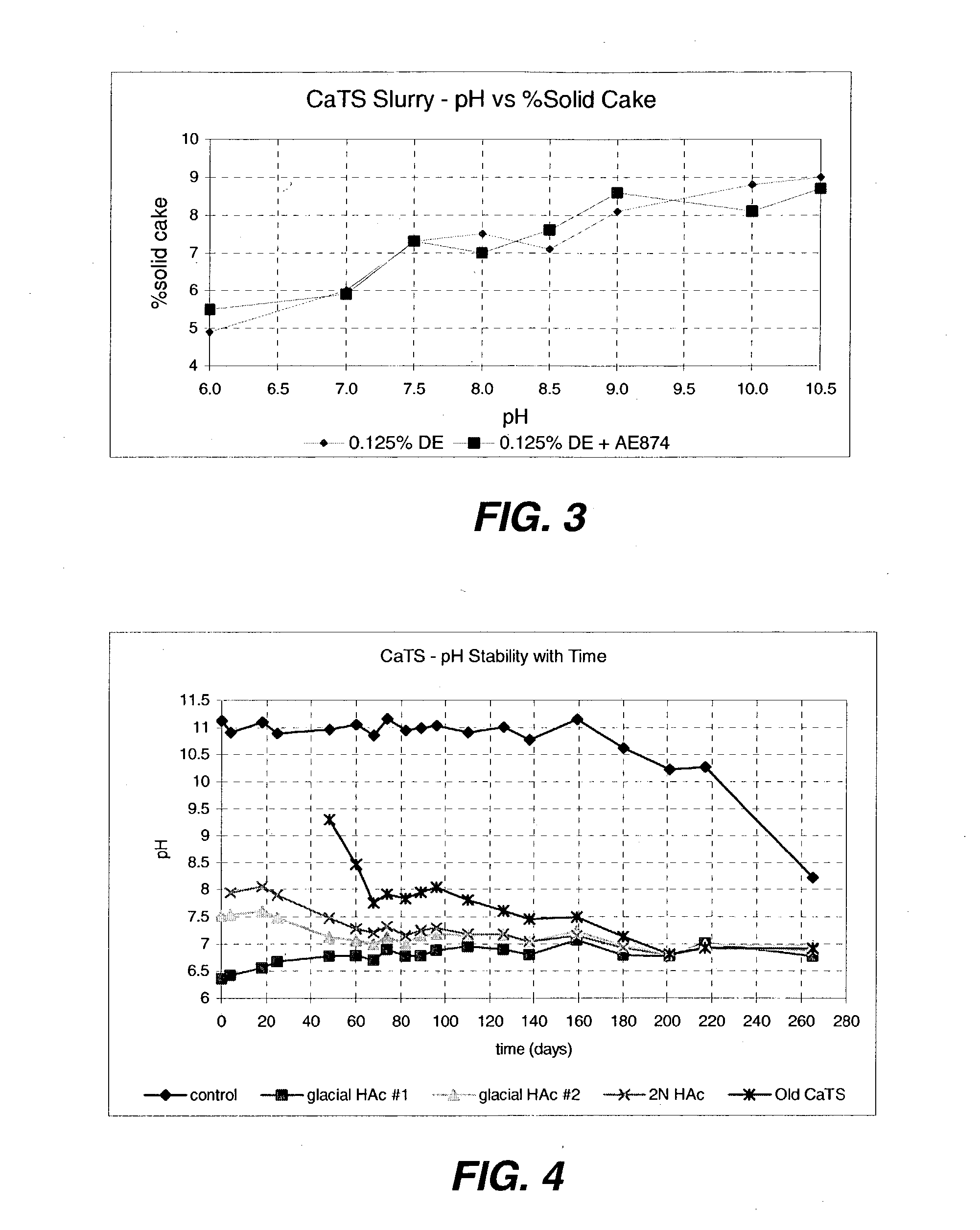
An efficient process to produce calcium thiosulfate (CaS2O3) from lime, sulfur and oxygen is described. By selecting appropriate process conditions such as mole ratios of lime to sulfur, temperature and pressure of the reaction process and the oxidation conditions, including rate and duration, the concentration of byproducts in the resulting suspension can be reduced to about 2% by weight or less. The solid particulate dispersion in the suspension tends to form a slimy solid suspension that is hard to filter if not treated properly. The suspension then can be acidified and treated with a flocculent. This agglomerates the solids into a floc that filters with ease. The resulting calcium thiosulfate is a clear liquid with concentrations achievable up to 29%.
Owner:TESSENDERLO KERLEY INC
Process for production of ammonium thiosulphate
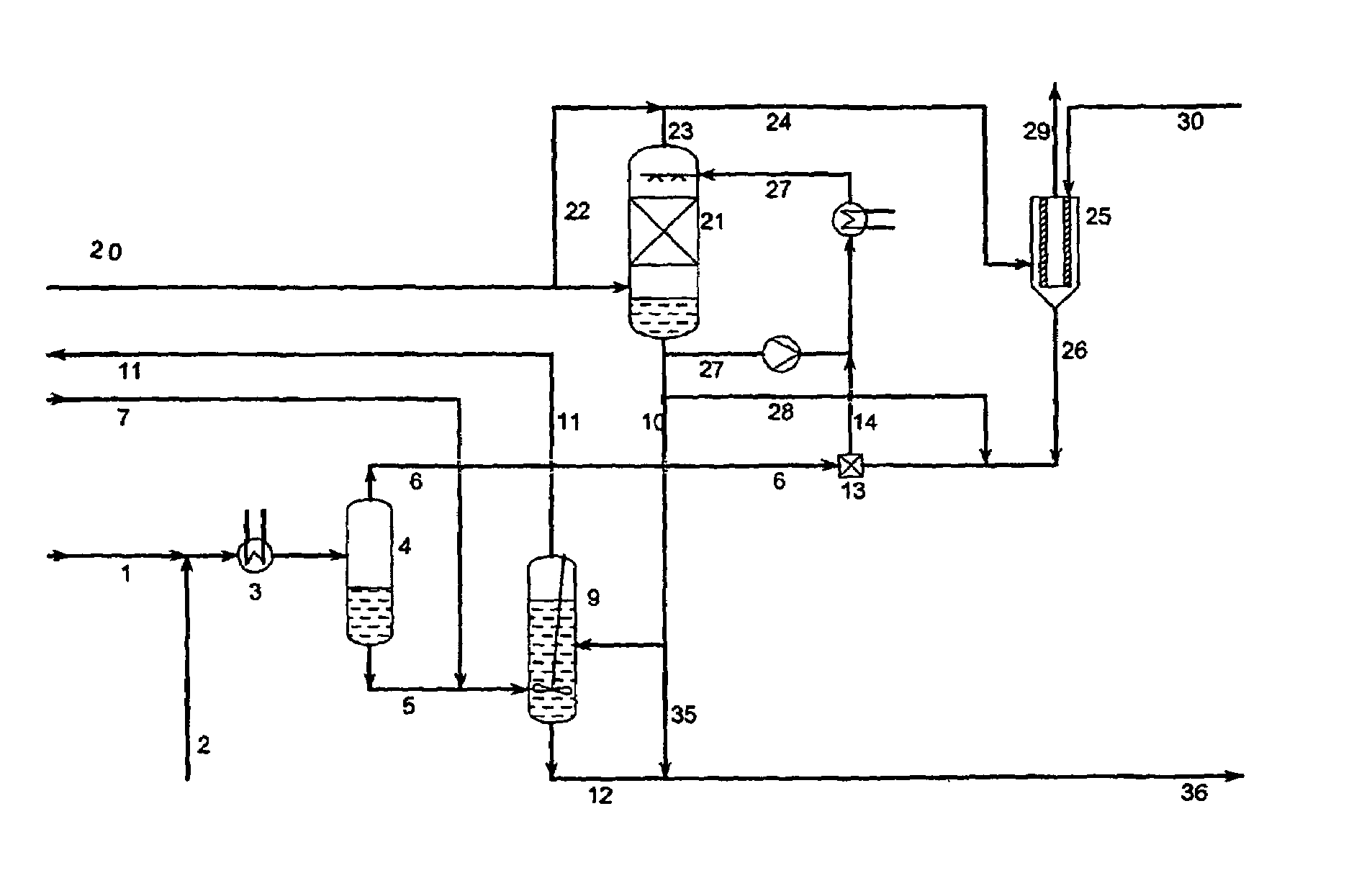
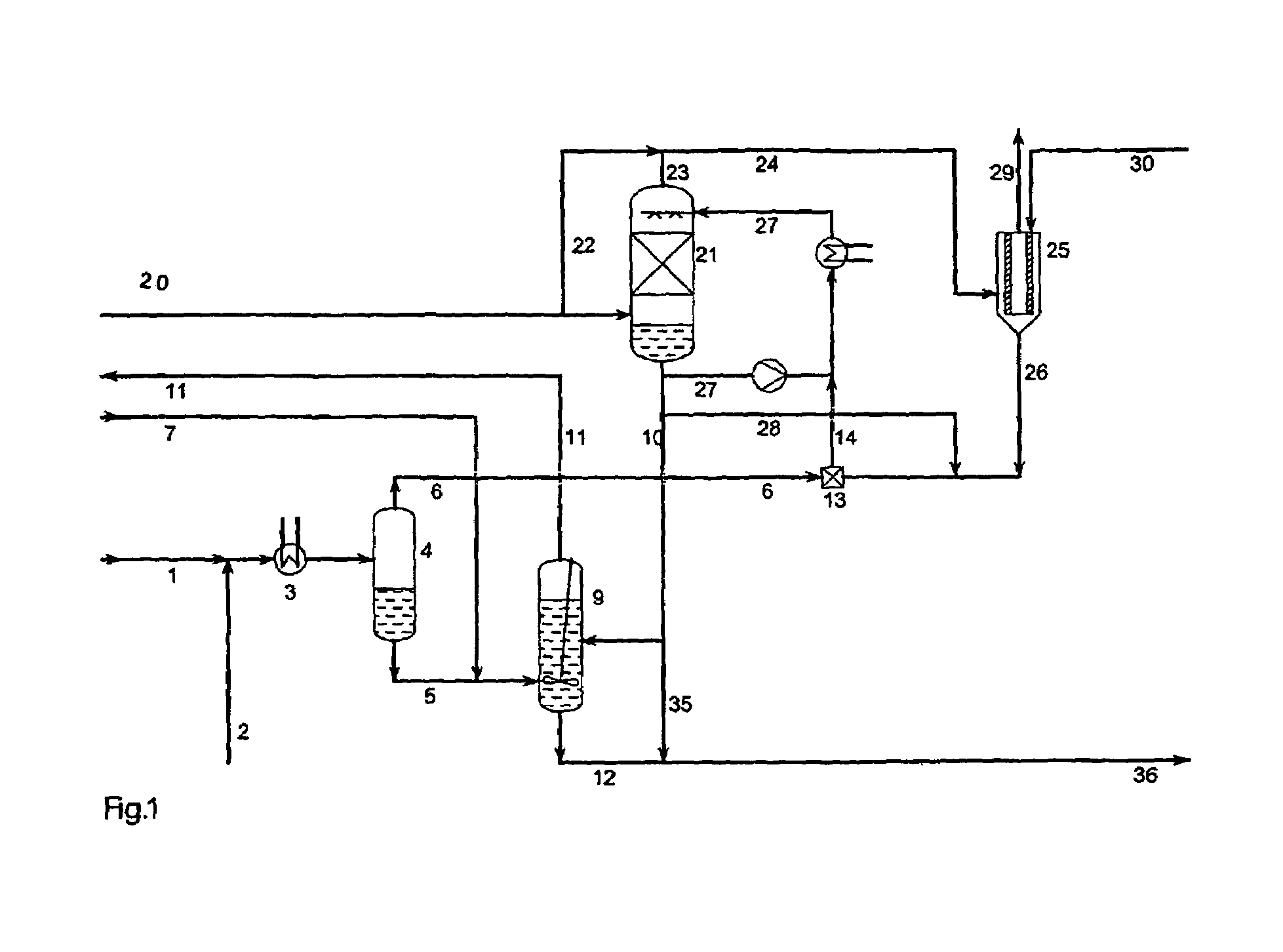
InactiveUS20030223930A1Speed up the processThiosulfates/dithionites/polythionitesSulfite preparationAqueous solutionAmmonium thiosulfate
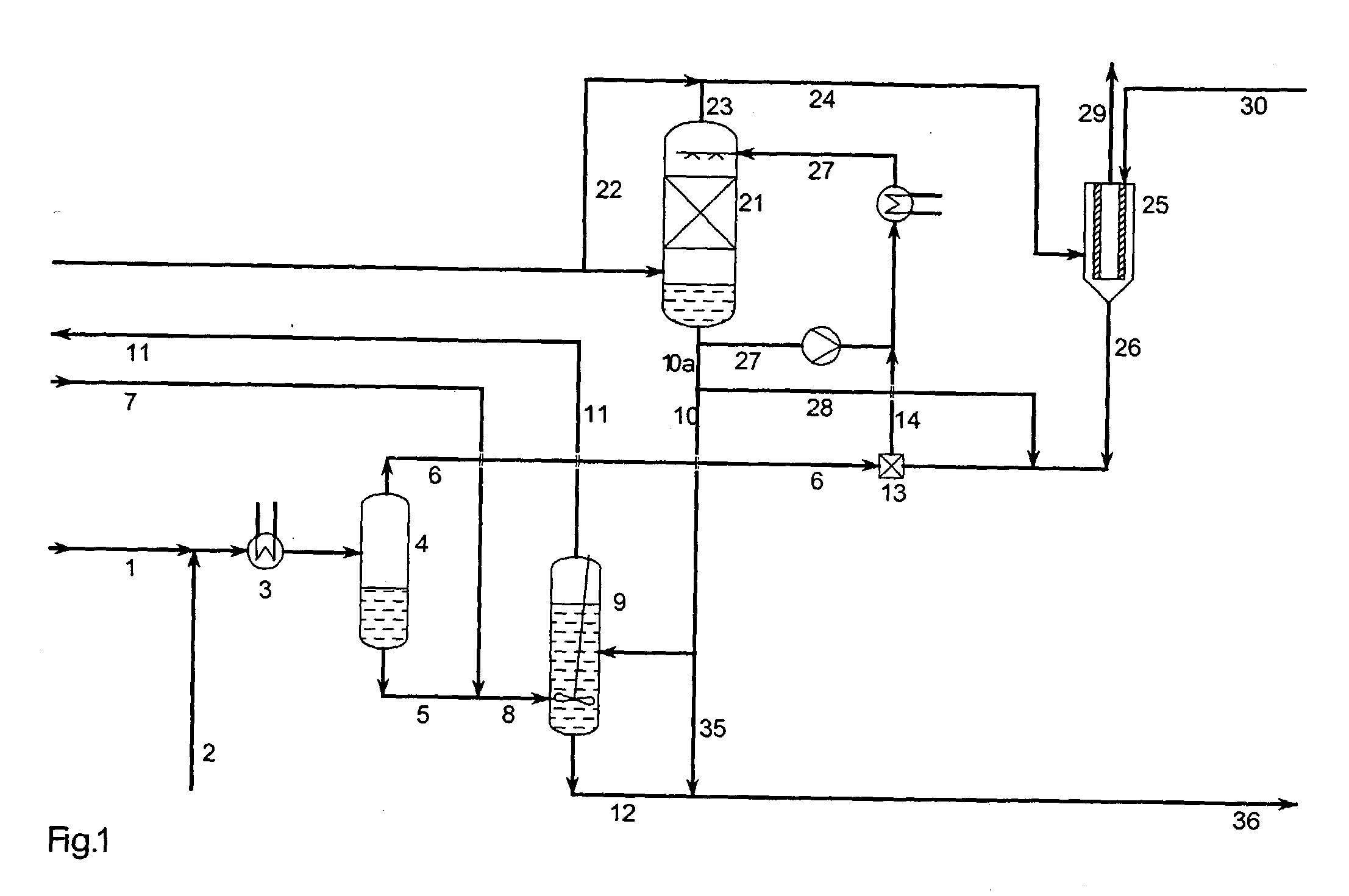
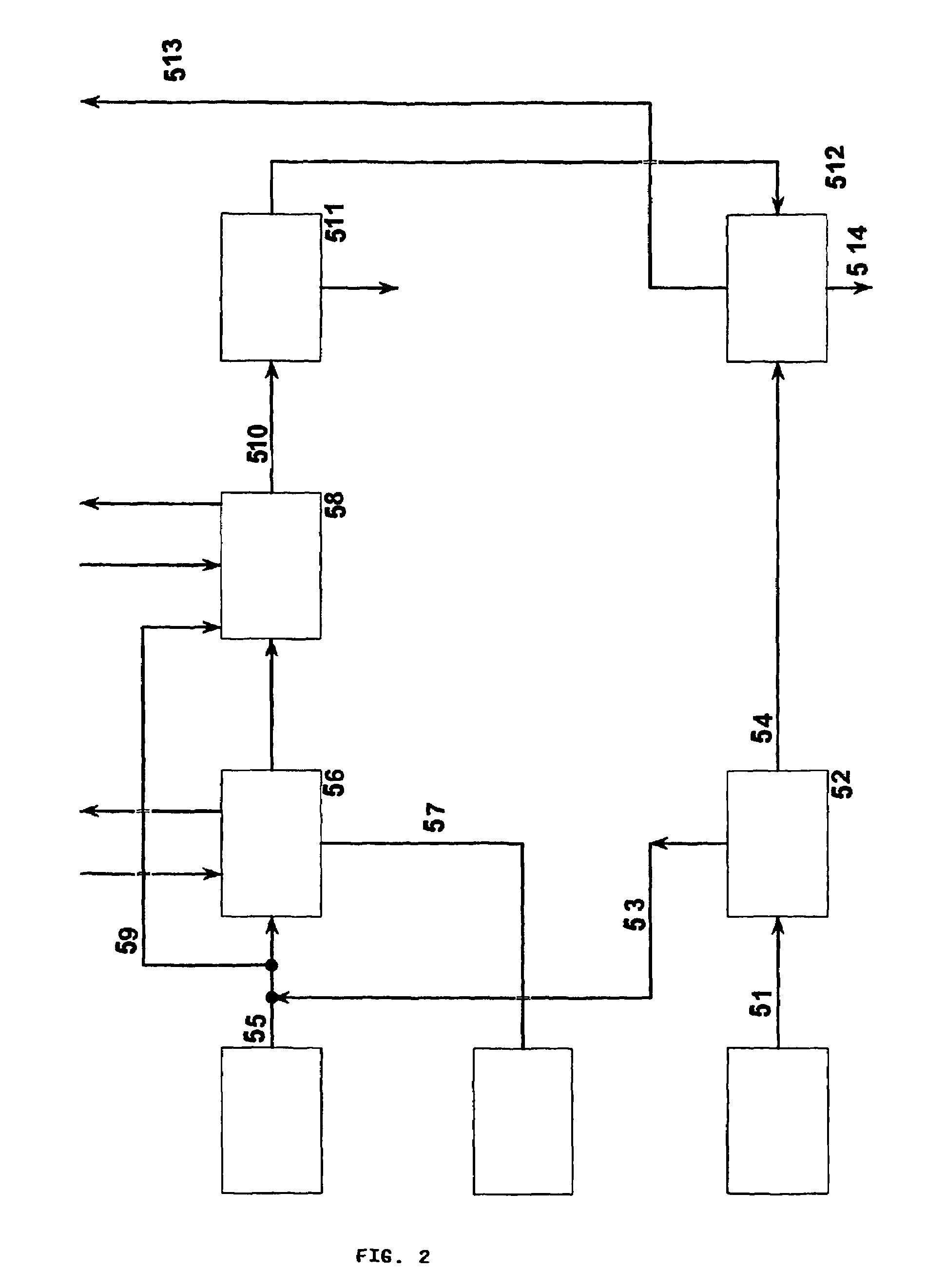
A process for continuous production of ammonium thiosulphate, (NH4)2S2O3 (ATS) from NH3, H2S and SO2 comprising steps of: (a) partial condensation in a partial condenser 4 of a first gaseous or partial liquid feed stream comprising H2O, H2S and NH3 with a molar H2S:NH3 ratio <0.4; (b) passing the aqueous condensate comprising NH4HS and NH3 from the partial condenser 4 to a reactor 9 in which said condensate is contacted with a third feed gas stream 7 comprising H2S and with an aqueous solution 10 comprising NH4HSO3 and (NH4)2SO3 under formation of an aqueous solution of (NH4) 2S2O3; (c) passing the gas stream comprising NH3 and H2S from the partial condenser 4 to a mixing device 13 in which said gas stream is completely dissolved in the water drained off from the aerosol filter 25; (d) passing a second feed gas stream 20 comprising approximately 2 / 3 mole SO2 per mole of NH3 contained in the first feed stream to a SO2 absorber 21 and the aerosol filter 25; (e) passing the aqueous solution produced in mixing device 13 to the SO2 absorber 21; (f) passing the off gas from the absorber 21 to the aerosol filter 25 and (g) adding to the aerosol filter 25 a balance amount of water required for obtaining approximately 40-65 wt % (NH4)2S2O3 in the aqueous of solution of (NH4)2S2O3 being withdrawn from the reactor 9.
Owner:HALDOR TOPSOE AS
Method for preparing calcium sulfide by reducing and decomposting gypsum through sulfur
InactiveCN101708825AImprove recovery rateAvoid Oxidation DisadvantagesMagnesium/calcium/strontium/barium sulfides/polysulfidesReduction rateCalcium sulfide
The invention discloses a method for preparing calcium sulfide by reducing and decomposting gypsum through sulfur, which is characterized in that: the method comprises the following steps: firstly, putting the gypsum into a reactor and raising the temperature to between 500 and 900DEG C for preheating 10 to 30 minutes at an inert atmosphere; and then introducing gaseous sulfur to perform reduction reaction with the gypsum for 0.5 to 2 hours to obtain a block, namely a calcium sulfide product, wherein the generated tail gas SO2 is used for producing sulphuric acid, and the gaseous sulfur accounts for 10 to 50 mol percent of mixed atmosphere in a reaction system. Because the method adopts a gas-solid reaction as a reaction mechanism, and the reaction is carried out at the inert atmosphere, the method has the advantages of low energy consumption, high reduction rate of over 95 percent, simple and mature process, short production period, easy control and convenient promotion.
Owner:SICHUAN UNIV
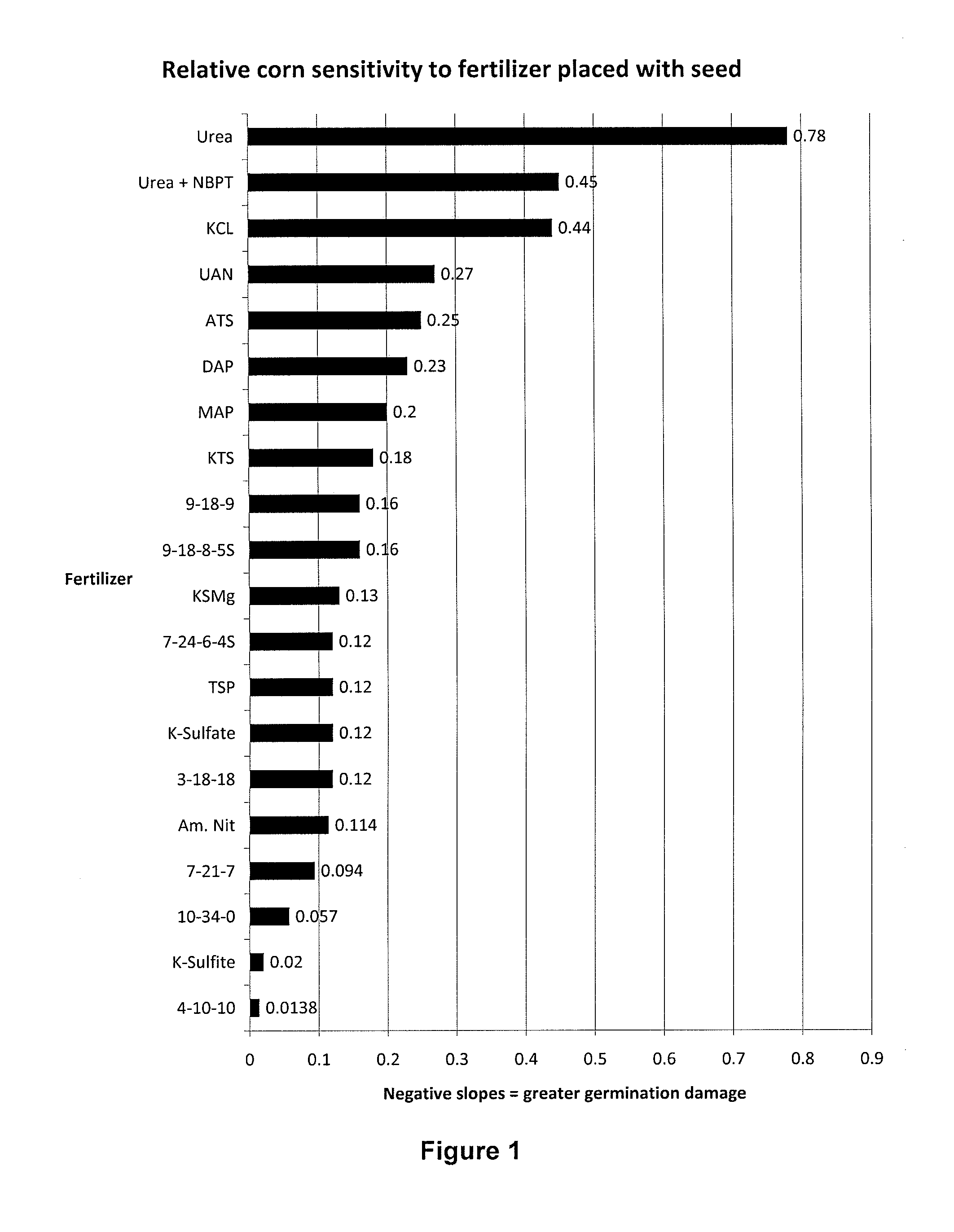
Potassium sulfite/potassium bisulfite (ks/kbs) liquid as starter, side-dress, broadcast, foliar and fertigation fertilizers
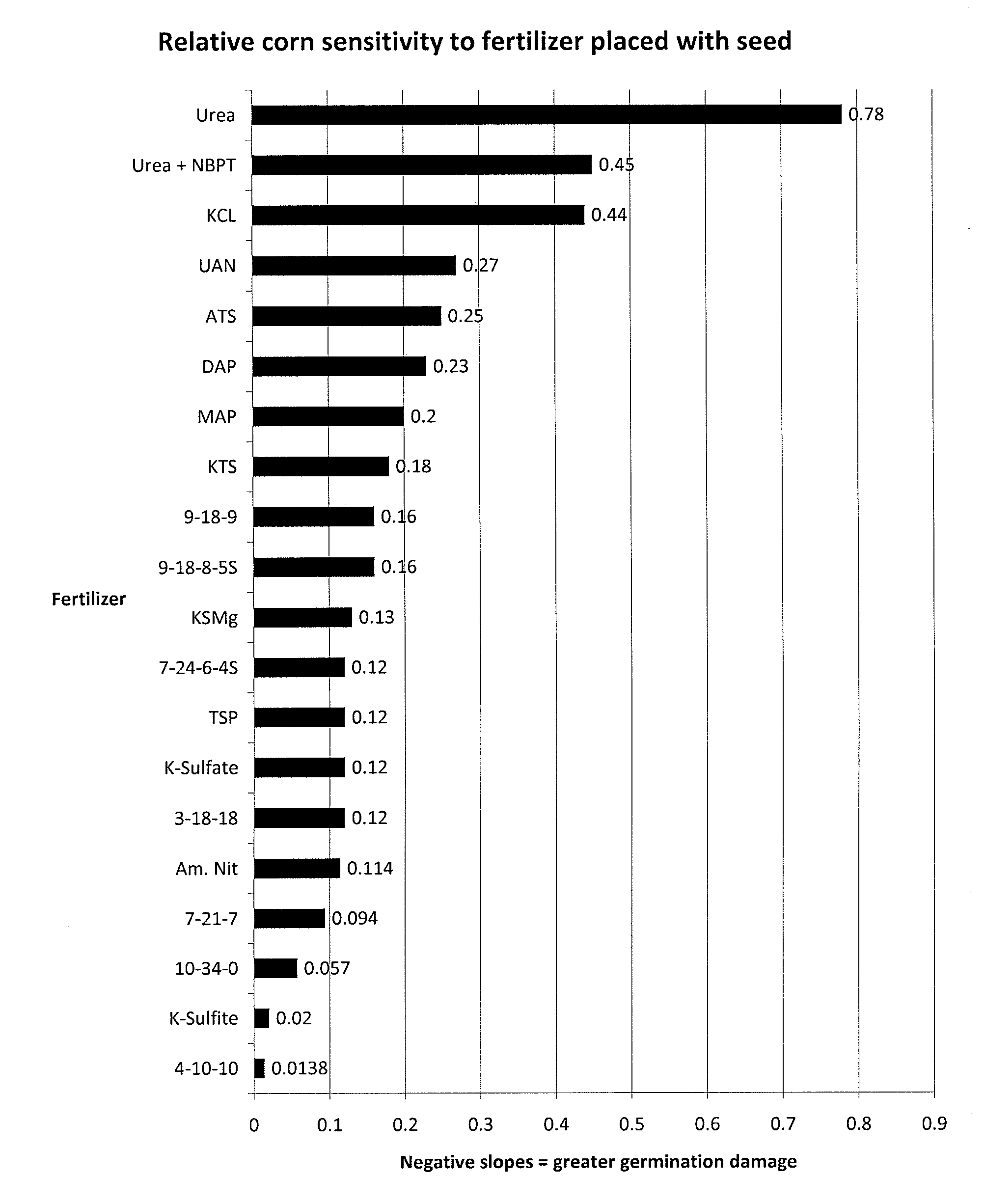
ActiveUS20120255335A1Low indexReduce drug damageBiocideSulfite preparationSulfur productPhytotoxicity
The present invention provides a new liquid fertilizer comprised of potassium sulfite and potassium bisulfite, with neutral to slightly alkaline pH, relatively lower salt index and potentially lower phytotoxicity damage compared to other sources of potassium and sulfur products applied in equal amounts as a starter fertilizer. More specifically, the present invention further relates to methods for fertilizing using a composition of potassium sulfite and potassium bisulfite, particularly as a starter fertilizer, in-furrow fertilizer, side dress fertilizer, and for foliar, broadcast, soil injection and fertigation applications. The fertilizer composition is comprised primarily of potassium sulfite (with the fertilizer grade of 0-0-23-8S).
Owner:TESSENDERLO KERLEY INC
Process for production of ammonium thiosulphate
InactiveUS7052669B2Thiosulfates/dithionites/polythionitesSulfite preparationAqueous solutionAmmonium thiosulfate
Owner:HALDOR TOPSOE AS
Method for producing calcium sulfide from calcium sulfate
ActiveCN101239706AReaction conditions are easy to controlImprove heat utilizationChemical industryMagnesium/calcium/strontium/barium sulfides/polysulfidesCelsius DegreeCoal
The present invention provides a method for producing sulfurated lime by calcium sulphate and a chemical producing process of sulfurated lime, especially producing sulphuric acid by calcium sulphate and chemical process of sulfurated lime in the production process of clinker. The invention has following steps: a. drying wet calcium sulphate in a drying crusher and material drying preheating system formed by a whirlwind preheater, and ascending the temperature of the material to 150-950 Celsius; b. adding grinded raw coal or coke which thickness is a 0.08mm square opening sieve (screen residue is below 30%) into raw material as a reducer; c. reducing calcium sulphate to sulfurated lime in the reactor containing calcium sulphate and C in the raw material under the impact of the reducing medium; d. mixing sulfurated lime generated by step C and unreacted calcium sulphate; e. So2 obtained by direct transferring reduzate to another reaction, controlling the mol rate of calcium sulphate and sulfurated lime between 6:1 and 1:6. The producing sulfurated lime method has a stable producing course, and a mass production.
Owner:YUNNAN YUNTIANHUA
Method of preparing metal chalcogenide particles
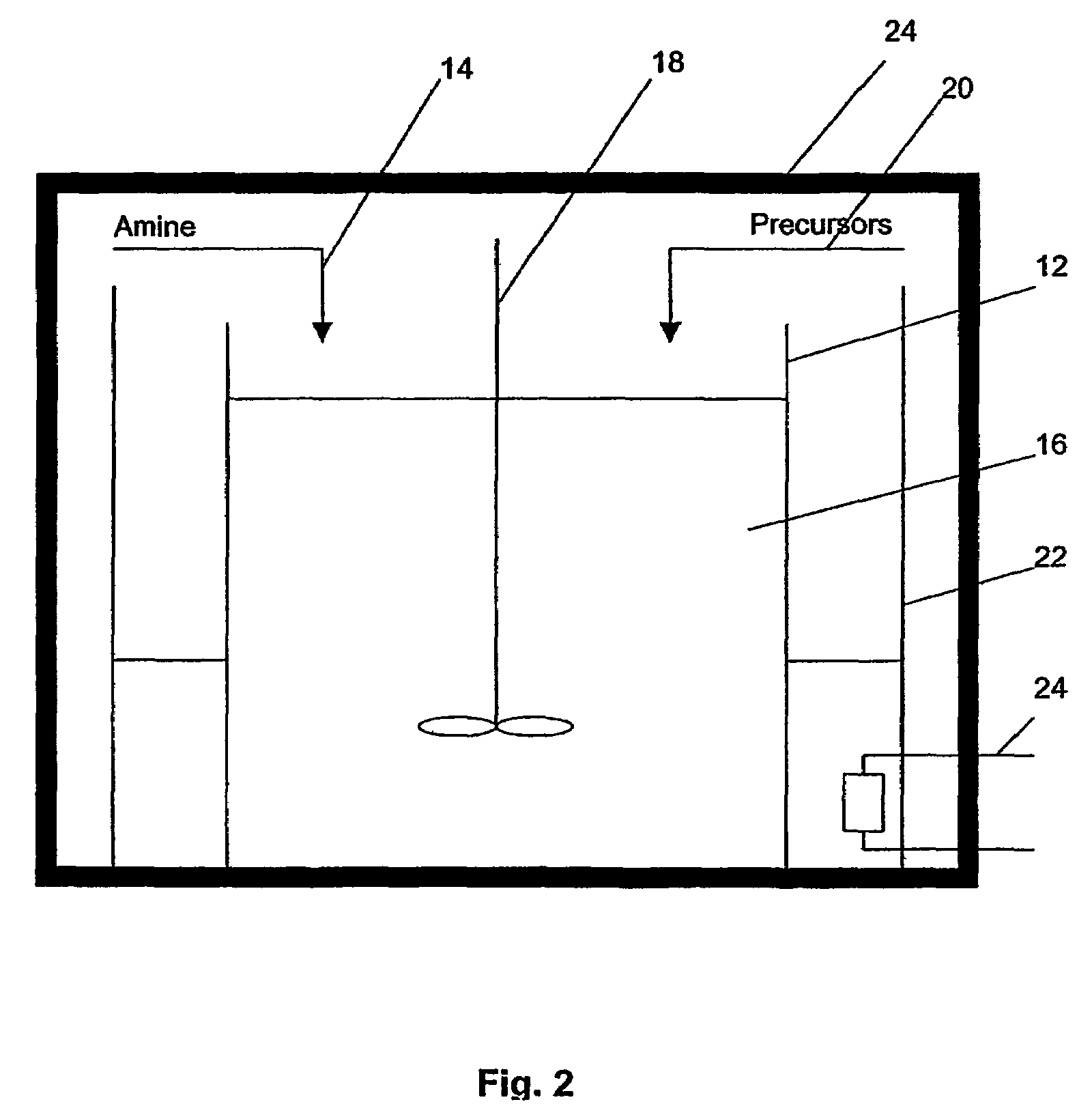
A method of preparing metal chalcogenide particles. The method comprising the step of reacting an amine and metal complex precursors. The metal complex precursors comprising a chalcogenide and an electrophilic group. The reaction forming metal chalcogenide particles substantially free of the electrophilic group.
Owner:NAT UNIV OF SINGAPORE
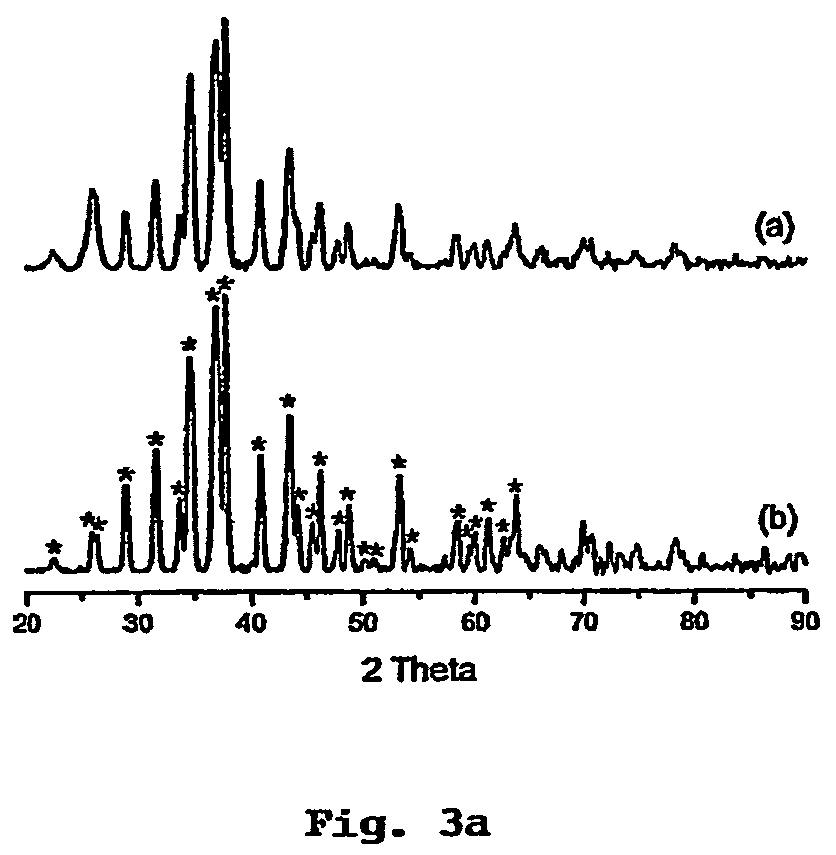
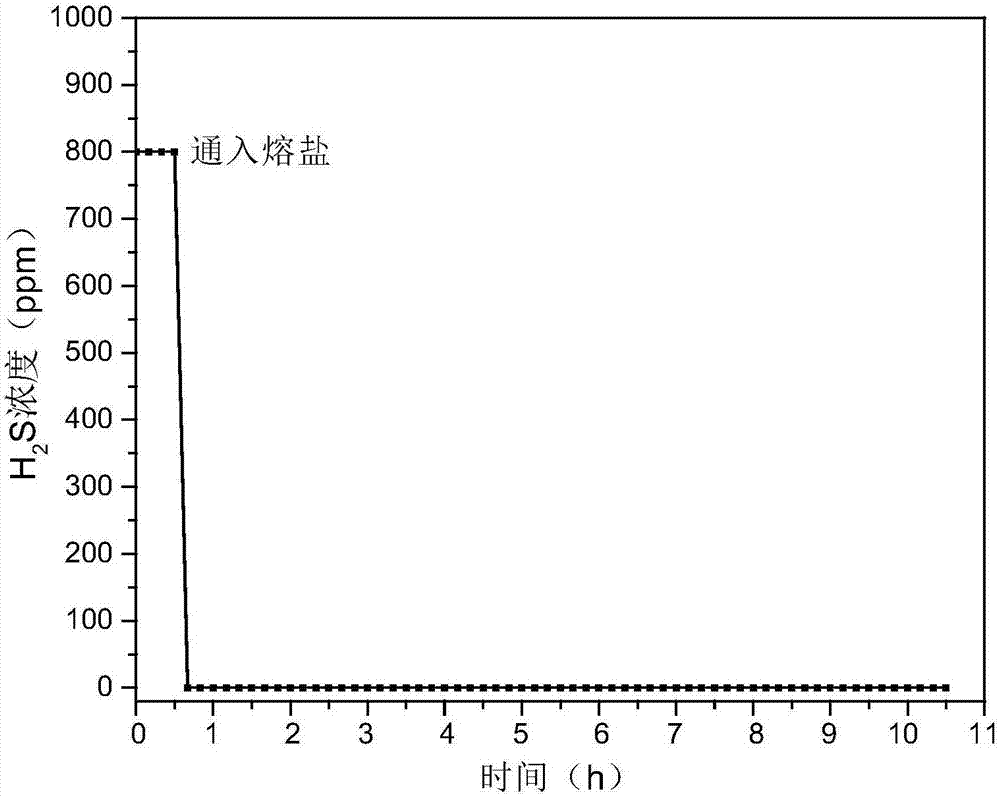
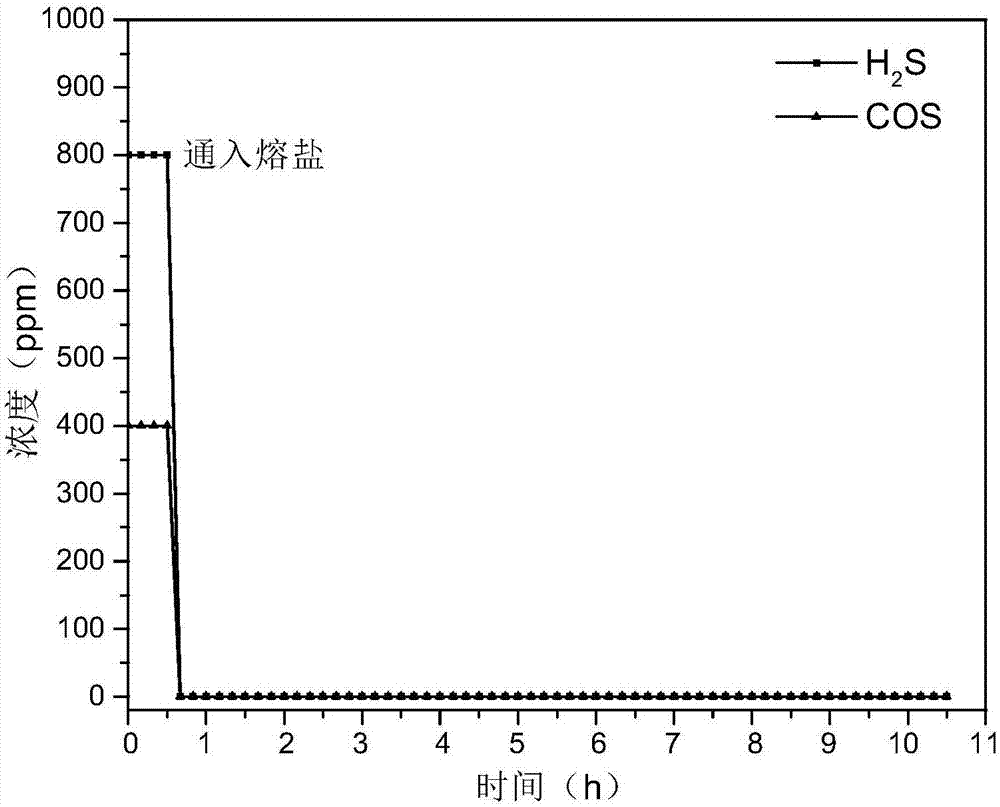
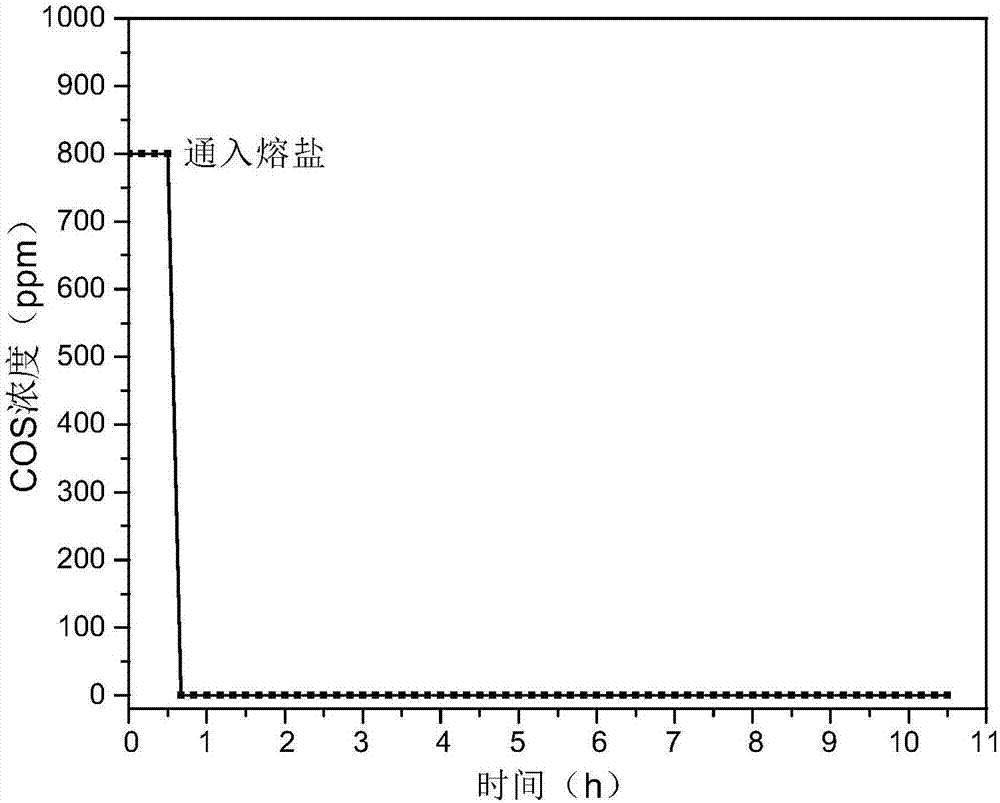
Hydrogen sulfide and carbonyl sulfide resource recycling method based on molten salt
InactiveCN107321165AAvoid heat lossGuaranteed Utilization EfficiencyGas treatmentDispersed particle separationBarium saltLiquid state
The invention discloses a hydrogen sulfide and carbonyl sulfide resource recycling method based on a molten salt. The method includes the following steps of (1) functional molten salt preparation, wherein a calcium salt or a barium salt is added into an alkali metal mixing molten salt system, then grinding and homogenizing are conducted, and the mixture is heated to melt to form the liquid functional molten salt; (2) hydrogen sulfide and carbonyl sulfide adsorption and enrichment, wherein the functional molten salt is heated to 450-750 DEG C, then carbon powder is added into the molten salt, and then gas with hydrogen sulfide and carbonyl sulfide is introduced into the molten salt to make the hydrogen sulfide, the carbonyl sulfide and the molten salt be in complete contact reaction; (3) sulfide recycling, wherein introduction of the gas with the hydrogen sulfide and the carbonyl sulfide is paused, the reacted molten salt system is supplemented with the calcium salt or the barium salt and stirred for mixing, then sulfide generated by reaction still stands for precipitation, and finally the sulfide is exported from the molten salt system at a high temperature for recycling. According to the method, the functional molten salt is used, instant purification of the gas with the hydrogen sulfide and the carbonyl sulfide at a medium-high temperature is promoted, long-term stable operation of the molten salt system is guaranteed, and resource recycling of sulfur is achieved.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for preparing synthesis gas from phosphogypsum
InactiveCN106118699ARaw materials are easy to getLow priceMagnesium/calcium/strontium/barium sulfides/polysulfidesSpecial form destructive distillationSyngasResource utilization
The invention discloses a method for preparing synthesis gas from phosphogypsum and belongs to the field of resource utilization of phosphogypsum. Lignite and phosphogypsum are taken as raw materials, after oxygen is removed from inert gas, calcination is performed, and the synthesis gas is prepared. The preparation process is simple and easy to implement, raw materials are industrial solid waste phosphogypsum and inferior lignite, the produced synthesis gas can be widely used for chemical feed gas, CaS can be obtained, and harmless, resourceful and high-value utilization of phosphogypsum is realized.
Owner:KUNMING UNIV OF SCI & TECH
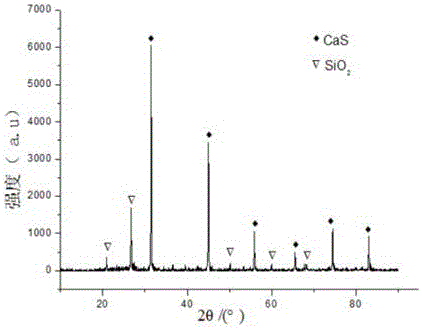
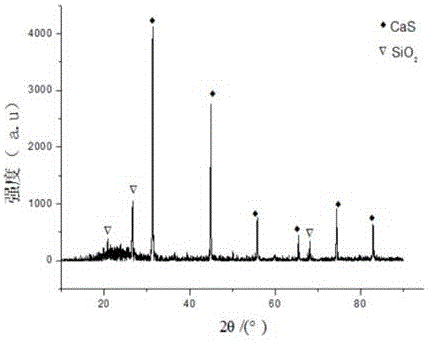
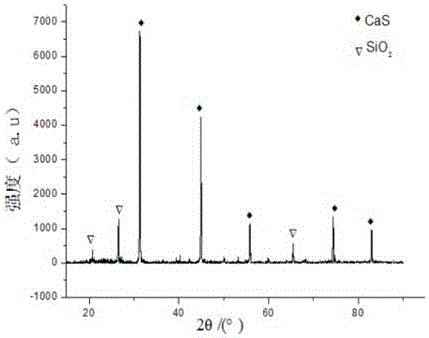
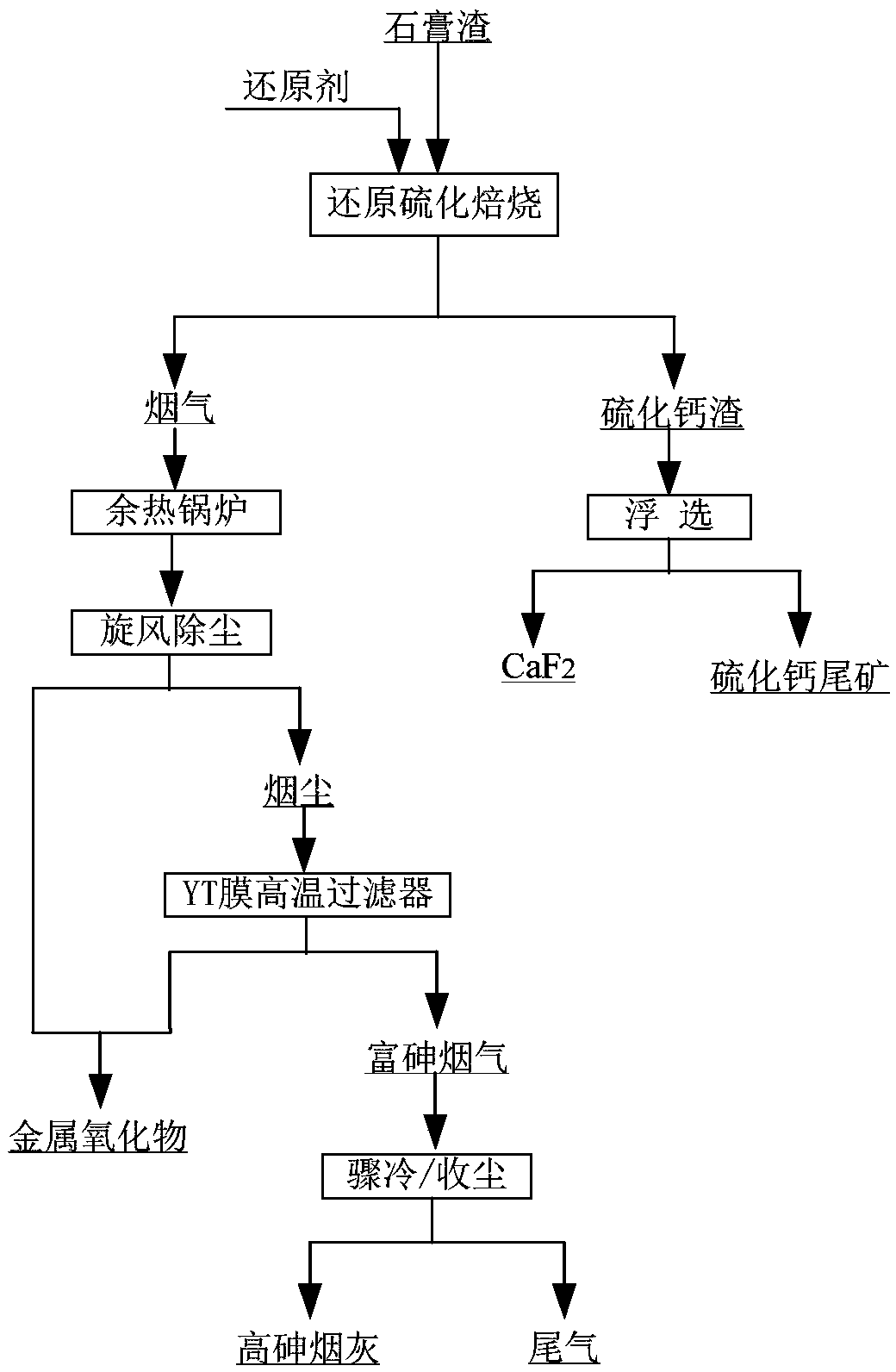
Recycling method of arsenic-containing gypsum slag
ActiveCN109052331ARealize recyclingAvoid influenceCalcium/strontium/barium fluoridesMagnesium/calcium/strontium/barium sulfides/polysulfidesSlagCalcium sulfide
The invention discloses a recycling method of arsenic-containing gypsum slag. The arsenic-containing gypsum slag is arsenic-containing gypsum slag produced in a waste acid neutralization process. Therecycling method comprises the following steps: (1) carrying out reduction roasting on the arsenic-containing gypsum slag by adopting a carbon reduction agent, so as to obtain arsenic-containing fluegas and roasting slag; (2) carrying out flotation separation on the roasting slag obtained in step (1), so as to obtain calcium sulfide concentrate and calcium fluoride slag. According to the recycling method disclosed by the invention, path opening of arsenic and fluorine in the gypsum slag and recycling of valuable metal are realized; influences caused by the fact that a lot of the arsenic-containing gypsum slag dangerous wastes are stacked and stored on the environment are solved, and the recycling method also has good economic and environmental benefits.
Owner:湖南锐异资环科技有限公司
Method for preparing caustic soda and coproducing calcium carbonate by using waste alkali liquid generated during ethylene cracking
InactiveCN102180486AEfficient governanceEfficient use ofCalcium/strontium/barium carbonatesMultistage water/sewage treatmentChemical reactionPetrochemical
The invention discloses a method for preparing caustic soda and coproducing calcium carbonate by using waste alkali liquid generated during ethylene cracking and relates to the technical field of comprehensive treatment and utilization of three wastes produced during ethylene cracking in petrochemical industry. The method comprises the steps of: heating waste alkali liquid generated during ethylene cracking, taking waste alkali liquid after all oil and soap are melted and float on the waste alkali solution; reacting the sodium sulfide in the obtained waste alkali liquid with calcium hydroxide in a glass lining reactor, starting a stirrer so that the solution has chemical reaction to produce mixture solution mainly containing sodium hydroxide; filtering to obtain a white calcium carbonate filter cake, washing and drying, crushing, separating and packaging to respectively obtain calcium carbonate and calcium sulfide products; sending the obtained clear sodium hydroxide alkali liquid in a normal-pressure evaporator, evaporating excessive moisture till the solution is in a saturated state, and then discharging, cooling into a block, and packaging to obtain a blocky caustic soda product. The method not only can lower the treatment cost of organic chemical industry and recycle waste material to create economic benefits, and but also can directly eliminate pollution sources.
Owner:何侠
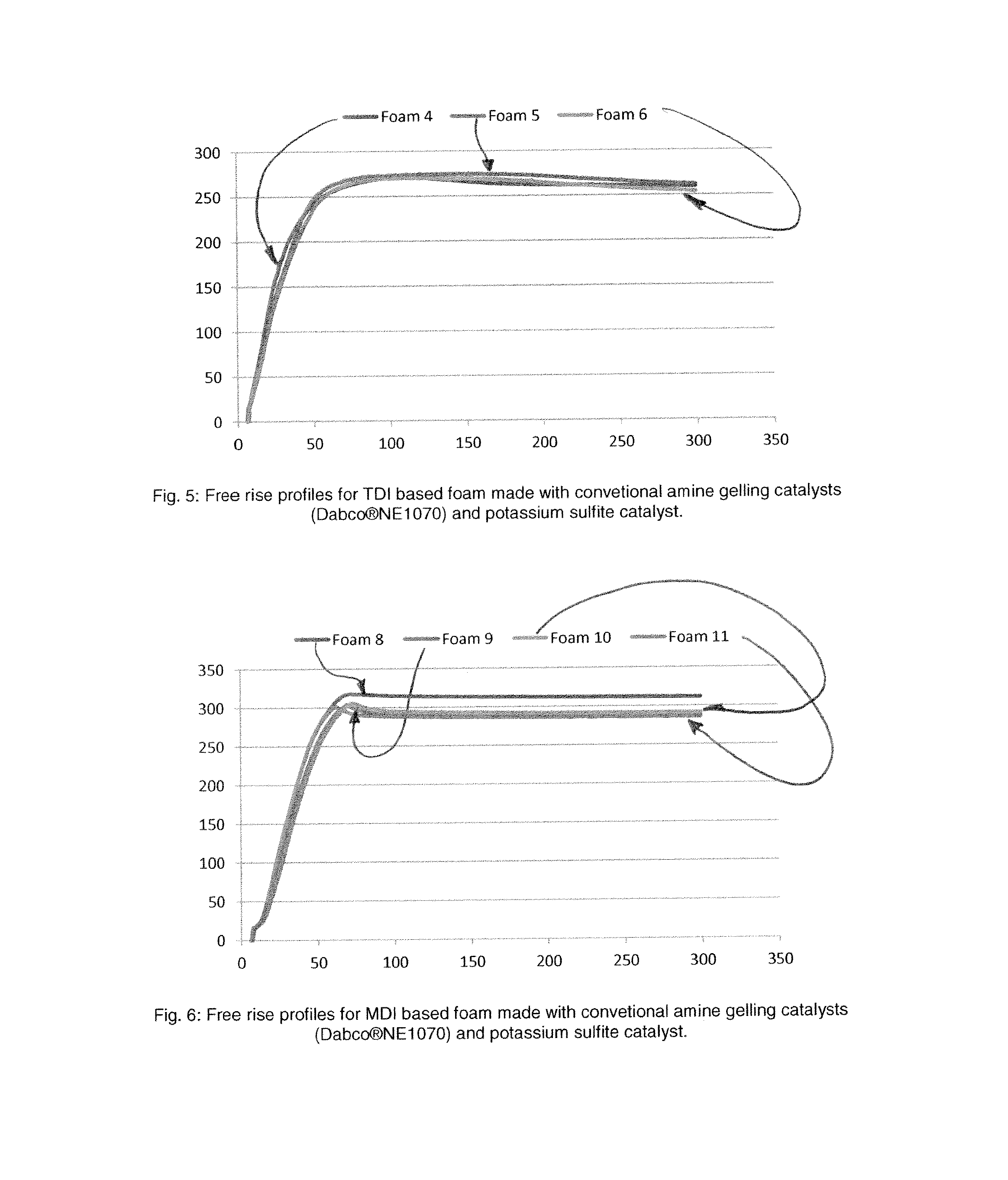
Polyurethane Catalysts from Sulfur Based Salts
ActiveUS20160102169A1Increase response rateReduction and elimination of emissionOrganic-compounds/hydrides/coordination-complexes catalystsMagnesium/calcium/strontium/barium sulfides/polysulfidesAdhesiveLacquer
This invention discloses the use of sulfite salts as catalysts to make polyurethane polymers. In particular, this invention discloses the use of metal salts such as alkali metal salts as well as alkyl ammonium salts such as tetralkyl ammonium salts as catalysts to make polyurethane polymers. The sulfite salts are useful to make a wide variety of polyurethane polymers and polyurethane foam polymer products such as flexible polyurethane foam polymers, rigid foam polyurethane polymers, semi-rigid polyurethane polymer, microcellular polyurethane polymer, and spray foam polyurethane polymer as well as any polymeric material that requires the assistance of catalysts to promote the formation of urethane and urea bonds such as those found in polyurethane emusions for paints, coatings, protective coatings, lacquer, etc as well as other polyurethane or polyurethane containing materials such as thermoplastic polymers, thermoplastic polyurethane polymers, elastomers, adhesives, sealants, etc. Examples of catalysts comprising the invention include sodium sulfite, potassium sulfite, lithium sulfite, tetramethylammonium sulfite and the like.
Owner:EVONIK OPERATIONS GMBH
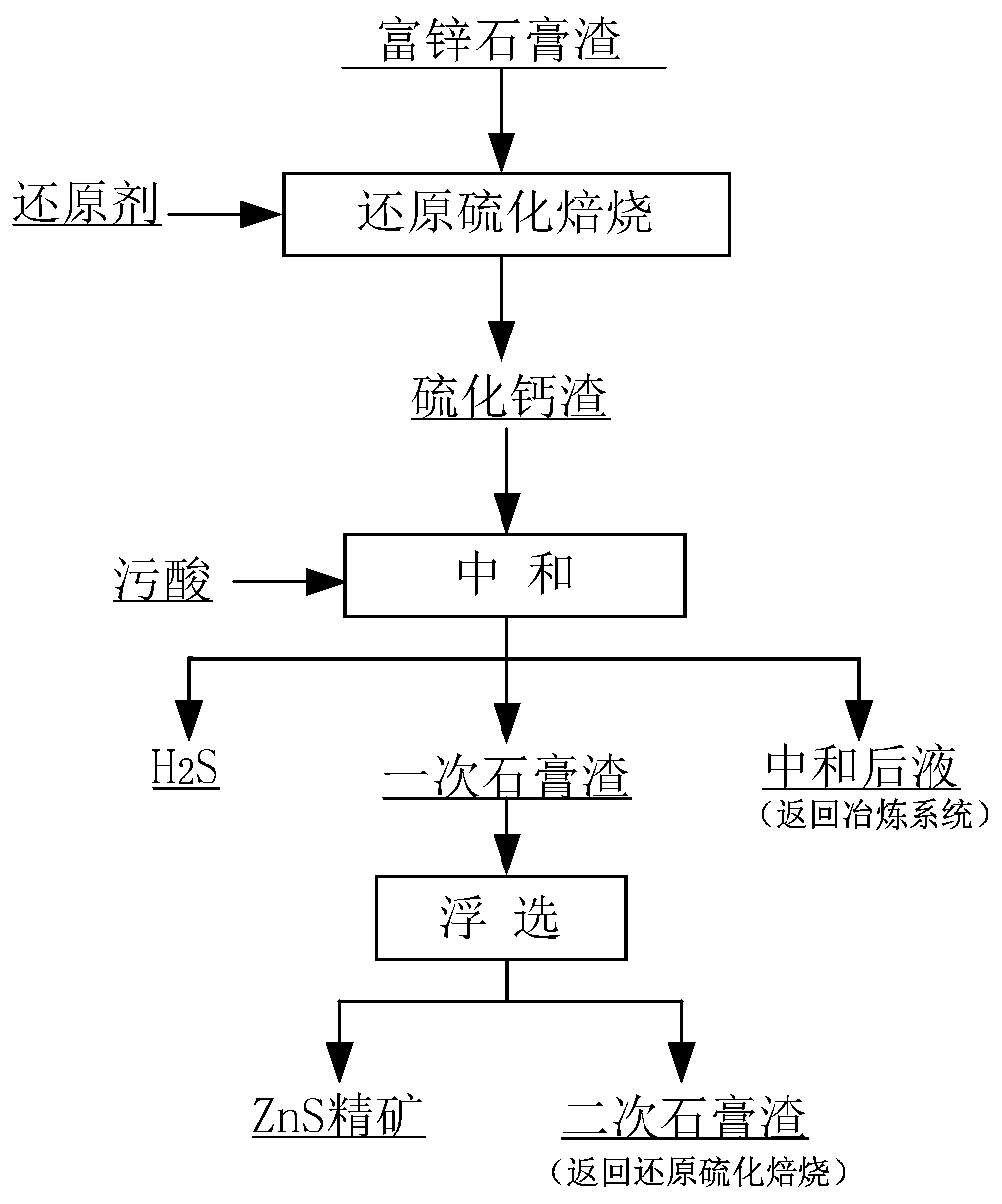
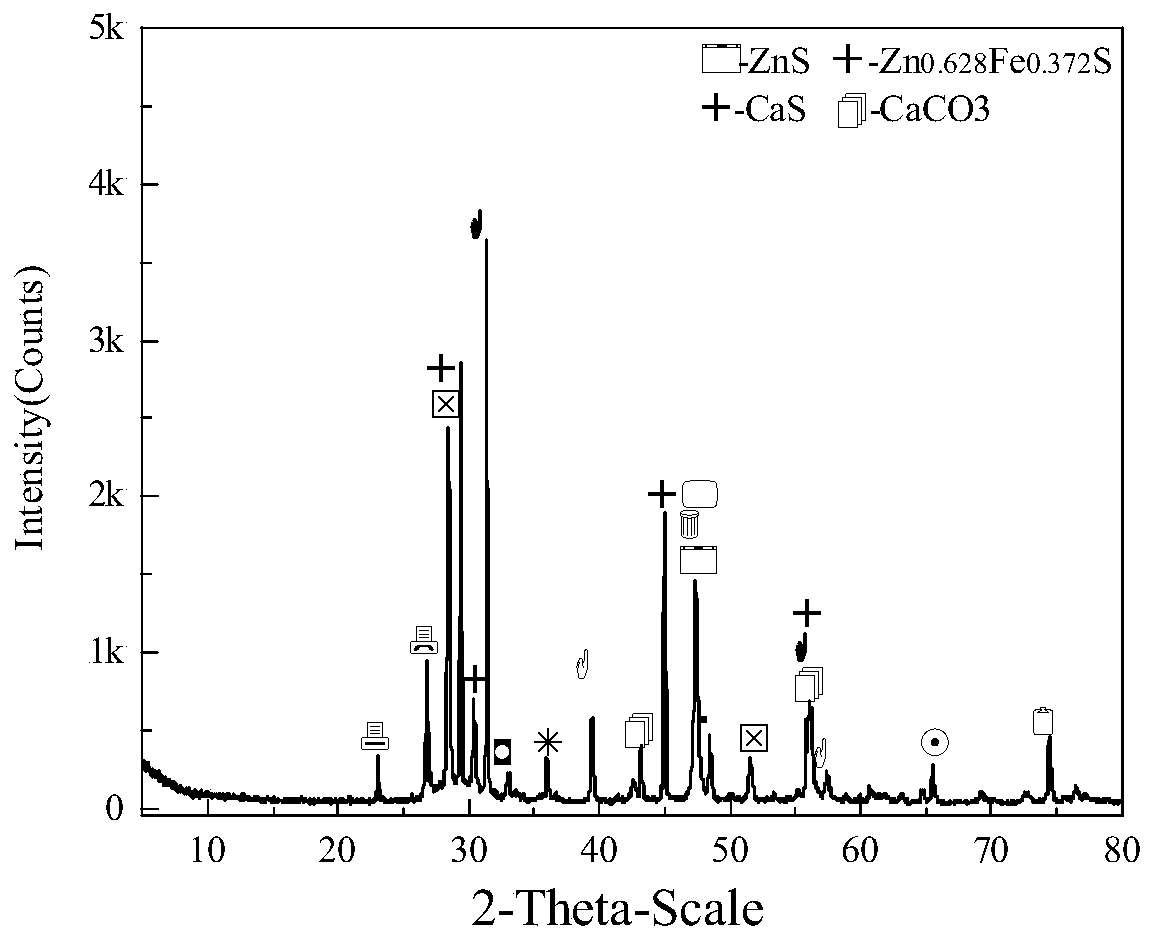
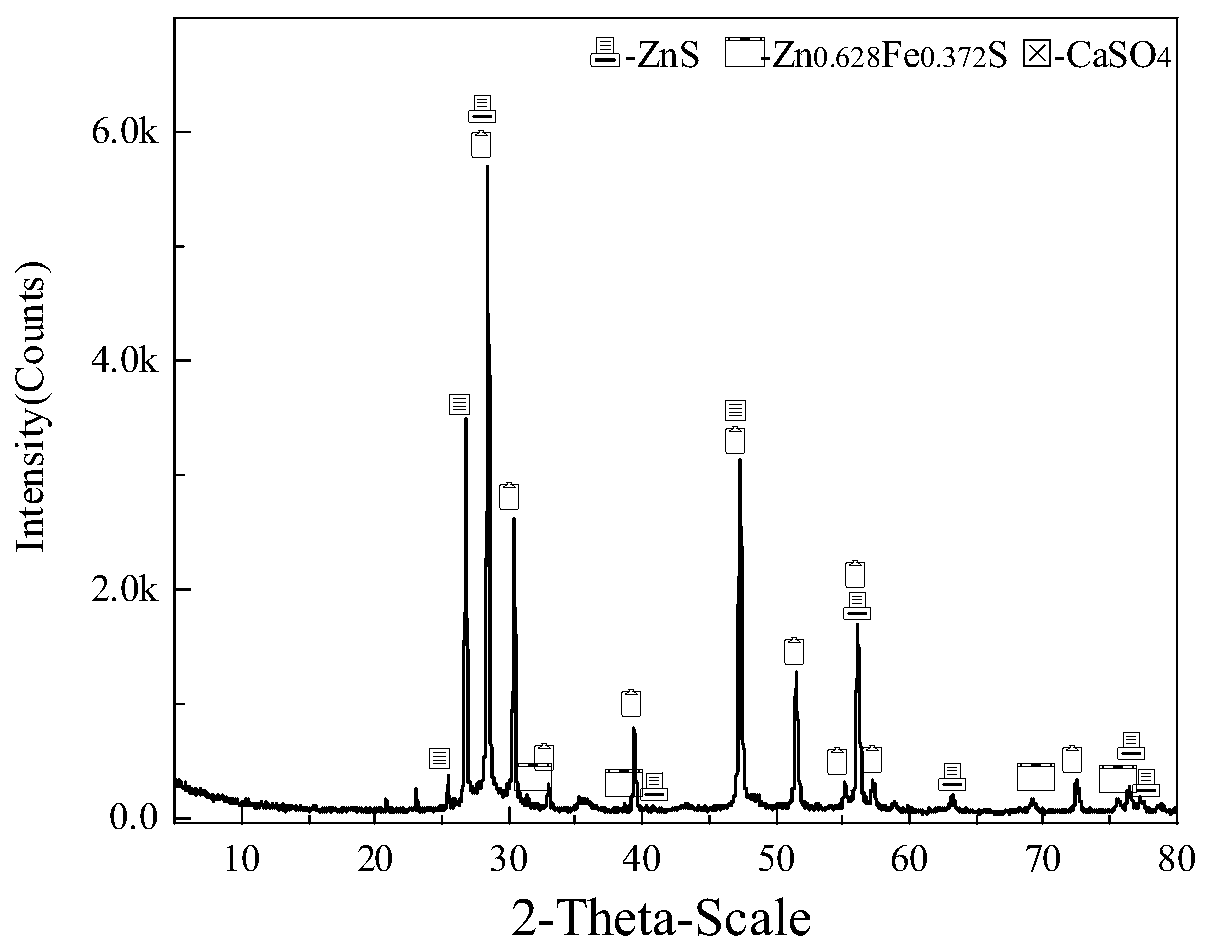
Method for comprehensively recycling zinc-rich gypsum slag resources
ActiveCN111020175AEffectively fixedAchieve recyclingMagnesium/calcium/strontium/barium sulfides/polysulfidesFlotationReducing agentChemistry
The invention discloses a method for comprehensively recycling zinc-rich gypsum slag resources. The method comprises the steps that reduction vulcanization roasting is carried out on zinc-rich gypsumslag and a carbonaceous reducing agent to obtain a roasting product containing calcium sulfide and zinc sulfide, a neutralizing reaction is conducted on the roasting product and zinc smelting acidic wastewater, hydrogen sulfide gas is recycled, and secondary gypsum slag and neutralized liquid are obtained; zinc sulfide concentrate is recycled from the secondary gypsum slag through a flotation method; and reduction roasting is conducted on flotation tailings to obtain a calcium sulfide product. According to the method, comprehensive recycling and utilizing of calcium, zinc and sulfur resourcesin the zinc-rich gypsum slag can be effectively achieved, the whole process is free of generation of secondary waste residues, the energy consumption is low, environmental friendliness is achieved, and the method has good application and popularization prospects.
Owner:CENT SOUTH UNIV
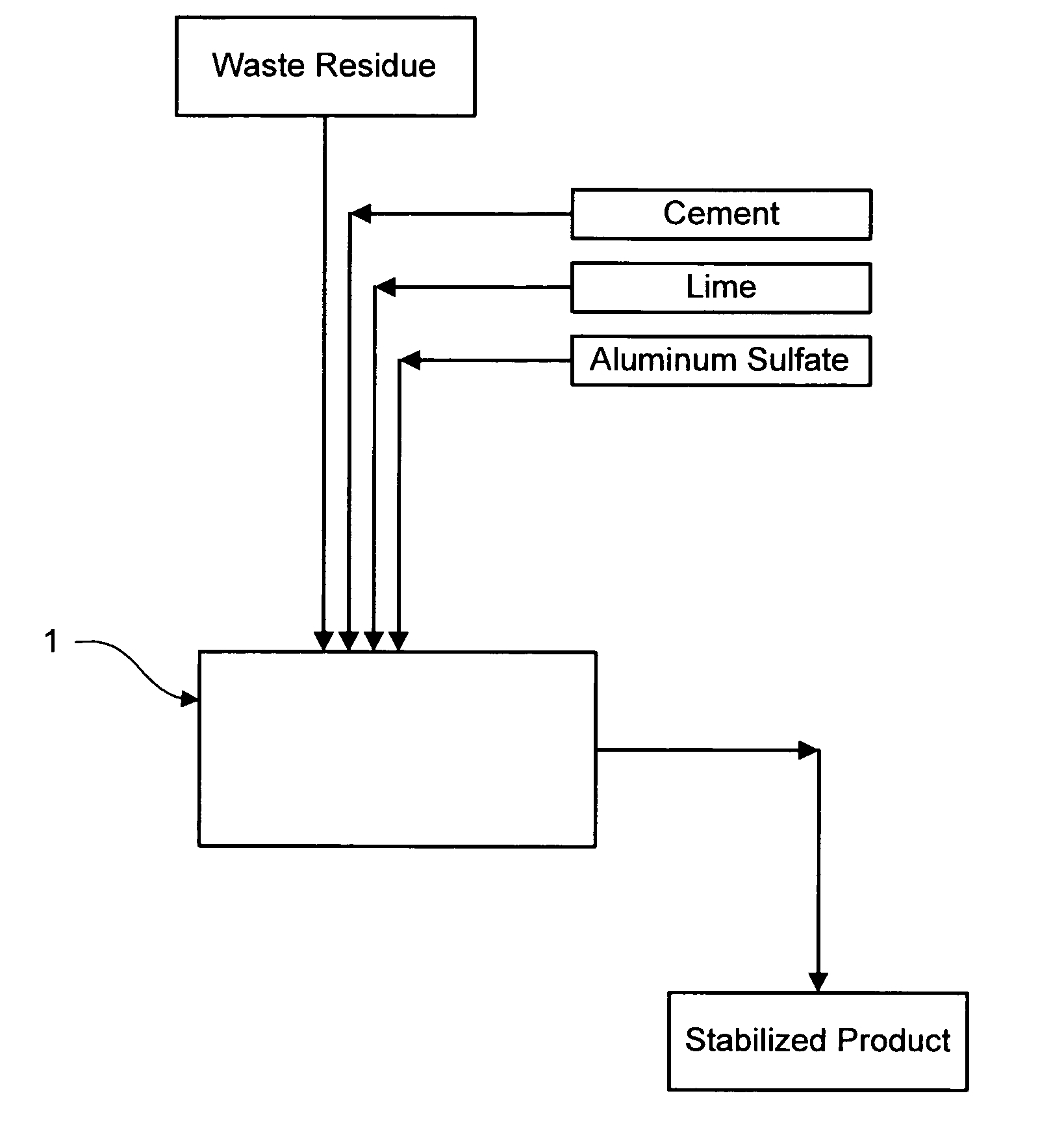
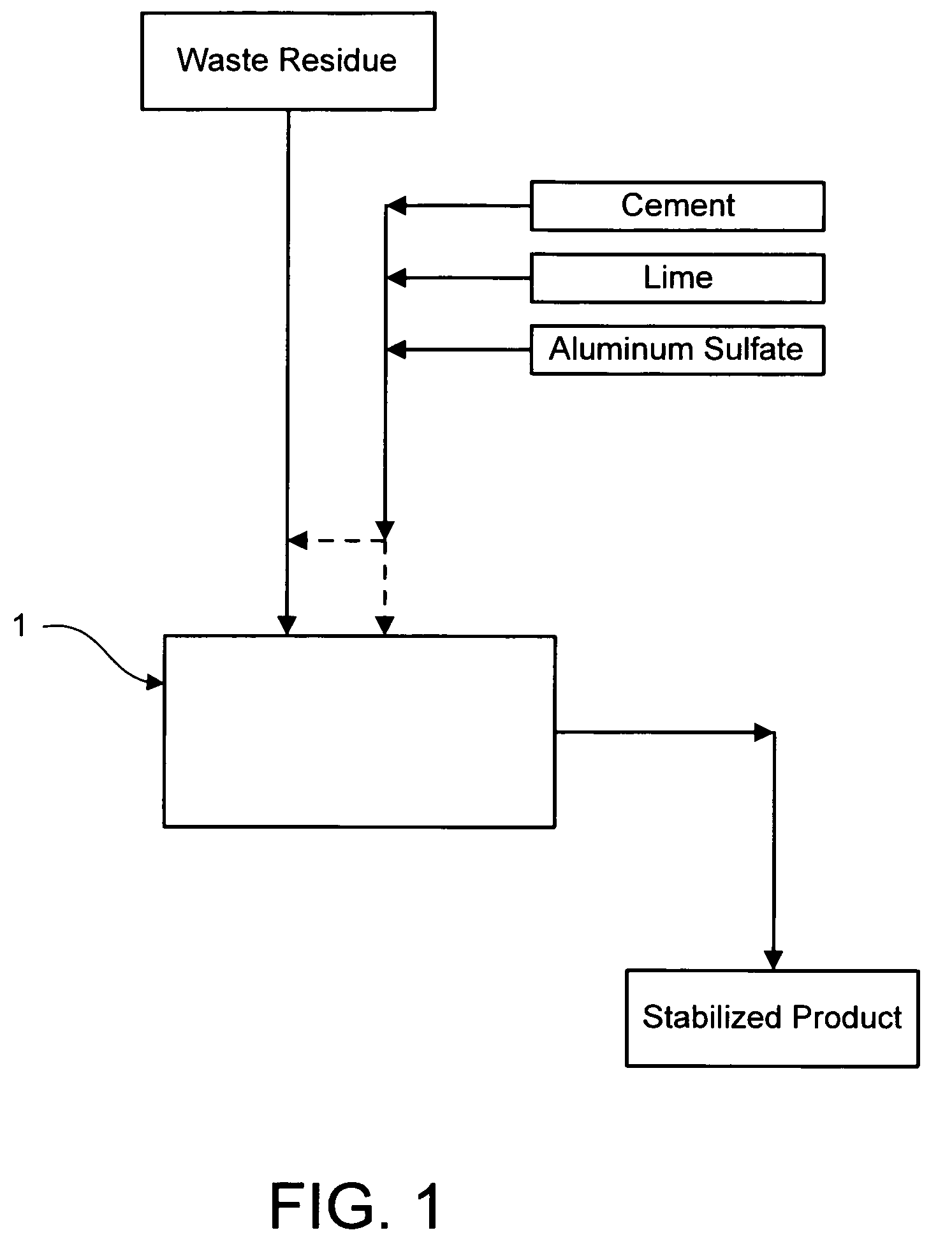
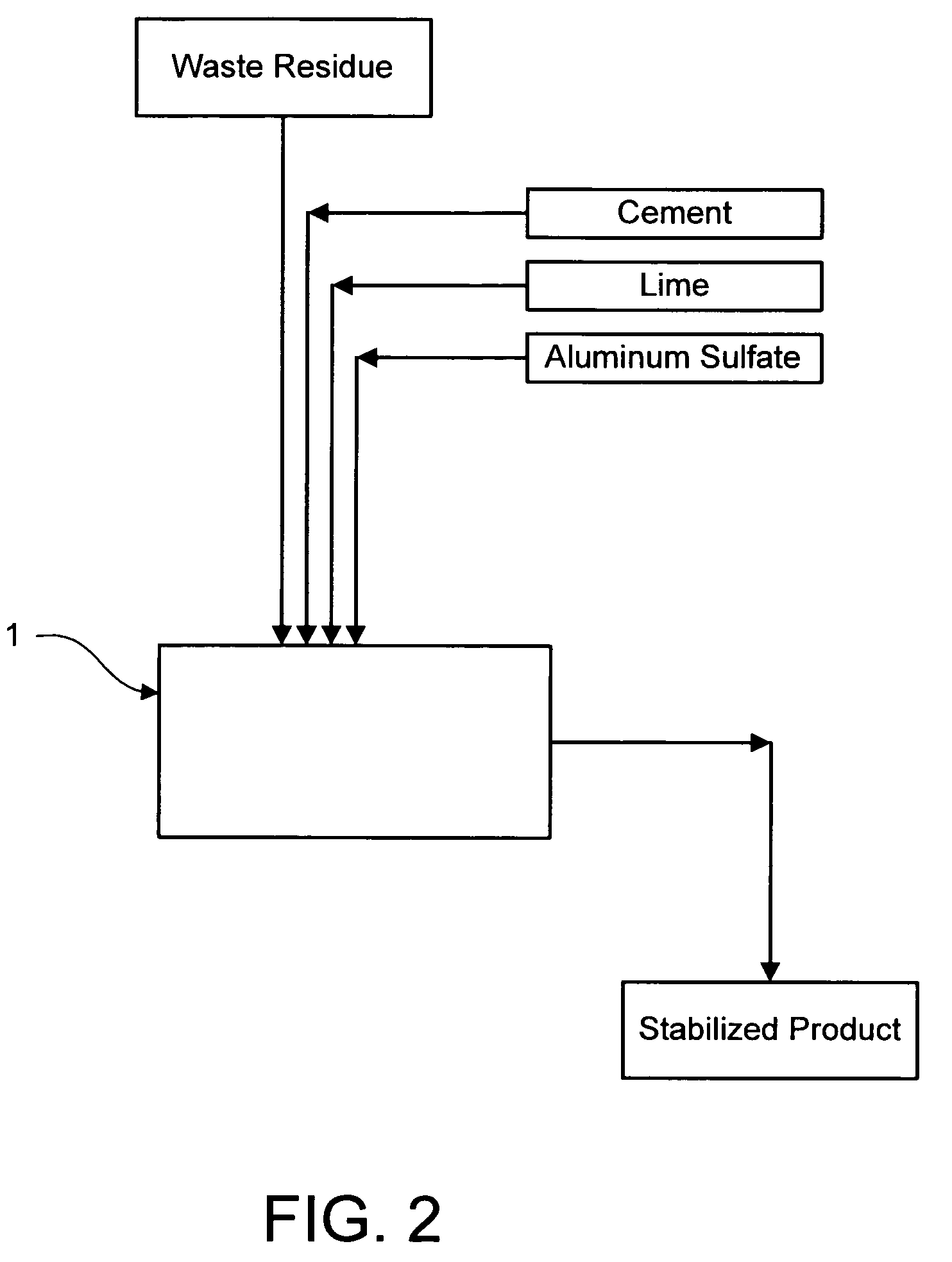
Method for chemically stabilizing waste materials containing multivalent oxyanions
Owner:REDOX TECH GRP LLC
Process and apparatus for preparing calcium thiosulfate solution
InactiveUS20040247518A1Thiosulfates/dithionites/polythionitesChemical/physical/physico-chemical stationary reactorsParticulatesSulfur
An efficient process to produce calcium thiosulfate (CaS2O3) from lime, sulfur and oxygen is described. By selecting appropriate process conditions such as mole ratios of lime to sulfur, temperature and pressure of the reaction process and the oxidation conditions, including rate and duration, the concentration of byproducts in the resulting suspension can be reduced to about 2% by weight or less. The solid particulate dispersion in the suspension tends to form a slimy solid suspension that is hard to filter if not treated properly. The suspension then can be acidified and treated with a flocculent. This agglomerates the solids into a floc that filters with ease. The resulting calcium thiosulfate is a clear liquid with concentrations achievable up to 29%.
Owner:TESSENDERLO KERLEY INC
Process and plant for the production of sulphuric acid
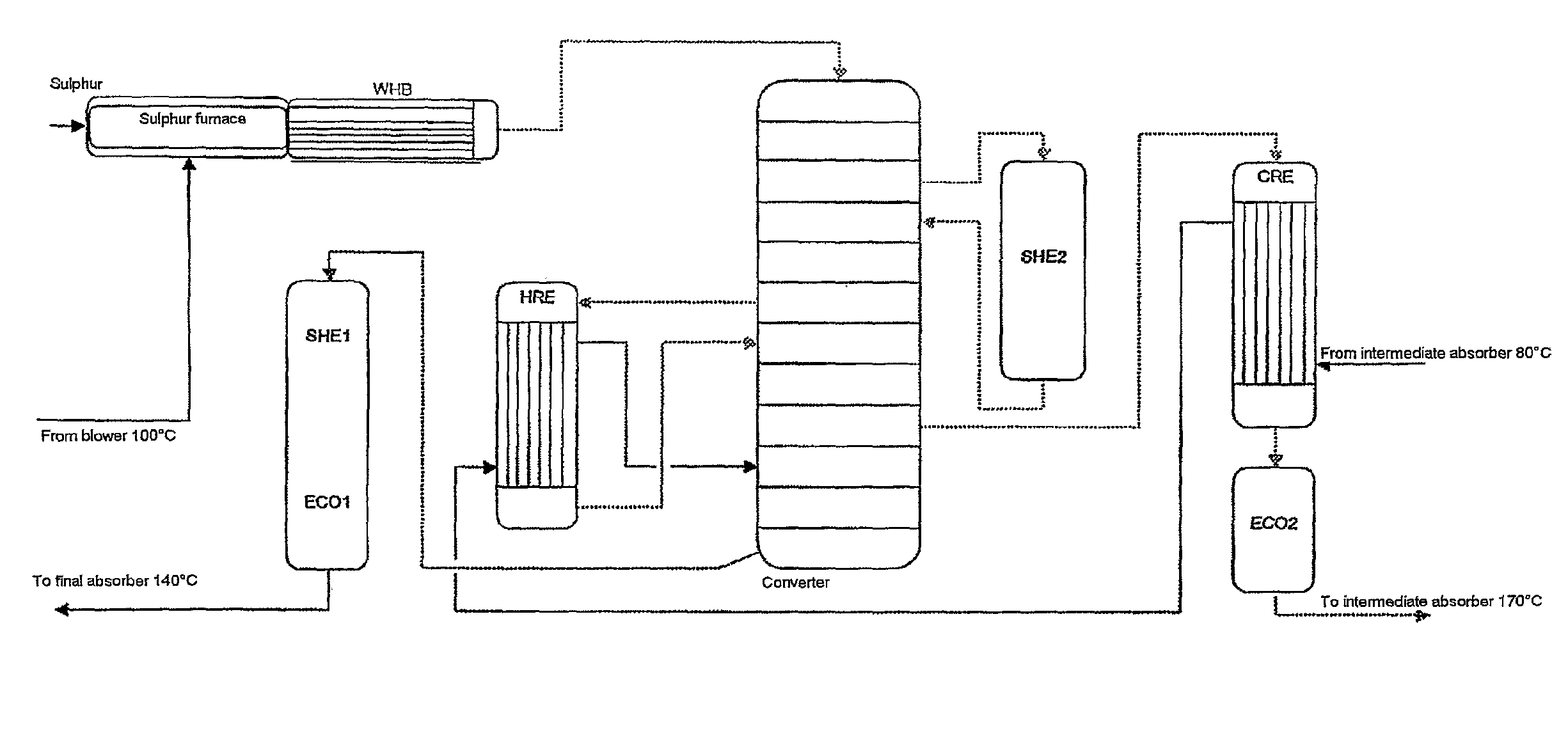
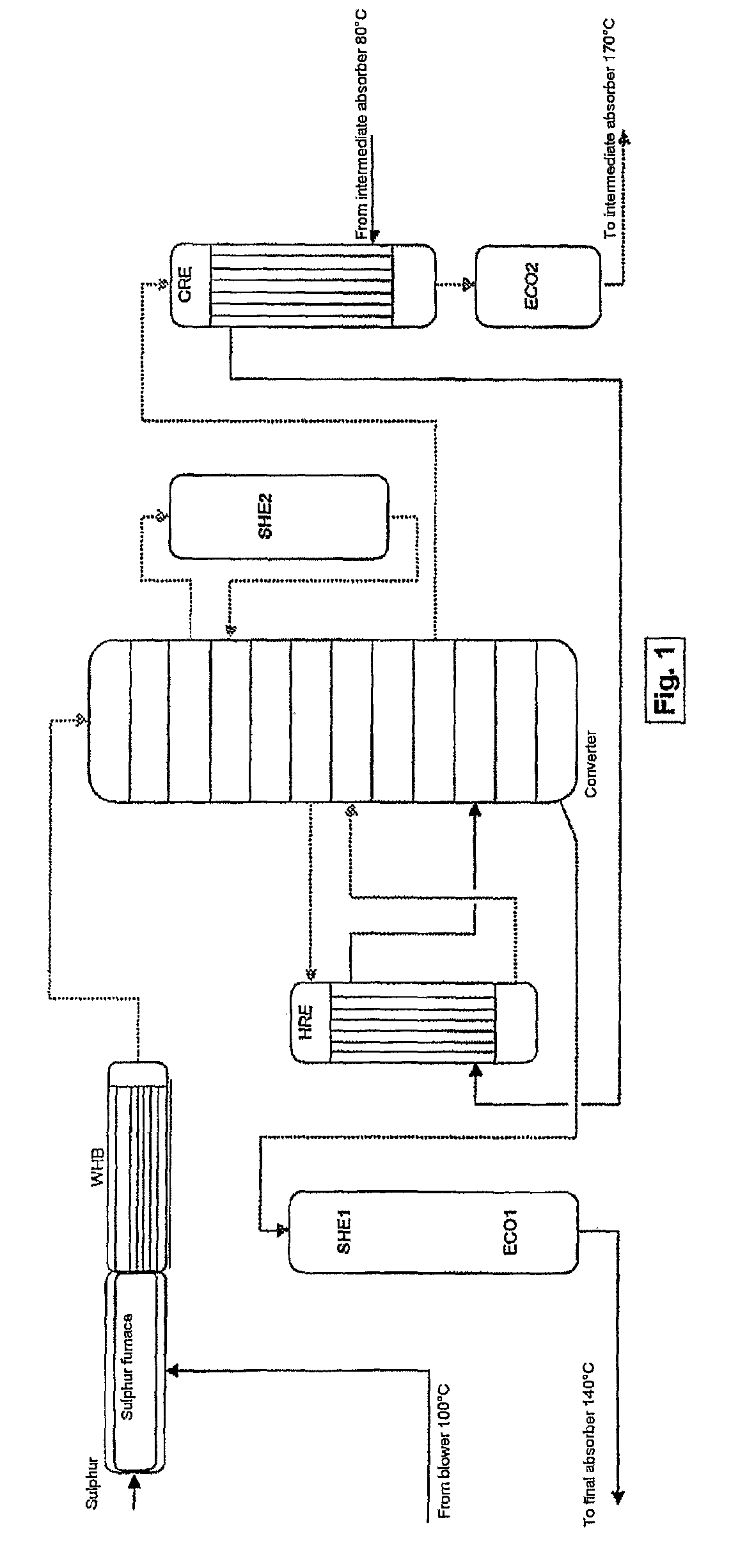
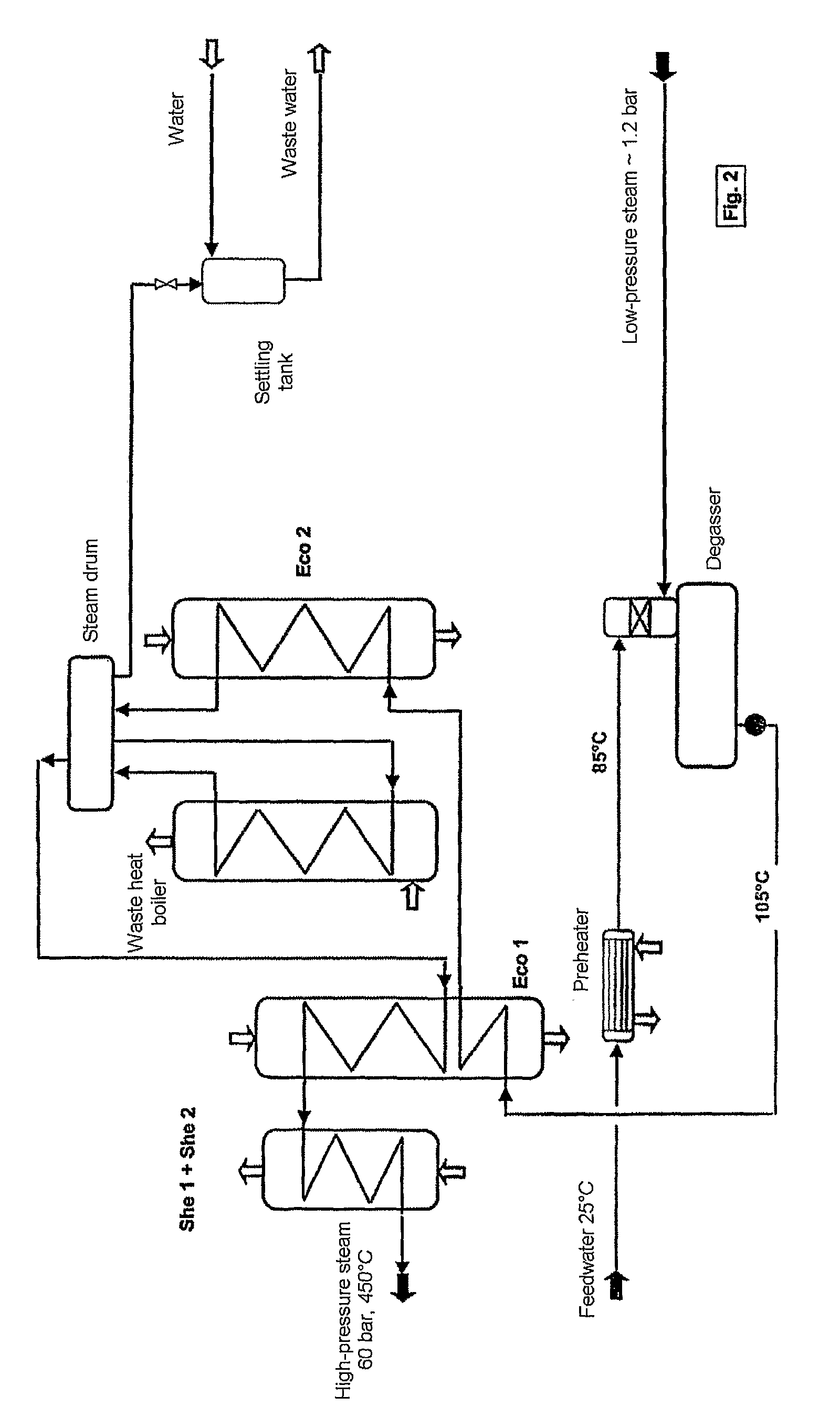
ActiveUS7837970B2Improve heat utilizationRaise the ratioEnergy inputSulfur preparation/purificationCatalytic oxidationChemistry
The invention relates to a process and plant for producing sulphuric acid by catalytic oxidation of SO2 to form SO3 in a converter with at least one contact stage, the SO3-containing process gas, after it has passed through at least one contact stage, being withdrawn from the converter and fed to an apparatus for recovering heat, in which steam is generated from feedwater by means of the heat of the process gas, and the process gas then being fed to an absorber, in which the SO3 is absorbed in sulphuric acid. To improve the utilization of heat during the production of sulphuric acid, the feedwater is fed to the heat recovery apparatus at a higher temperature than the process gas fed to the absorber.
Owner:METSO METALS OY
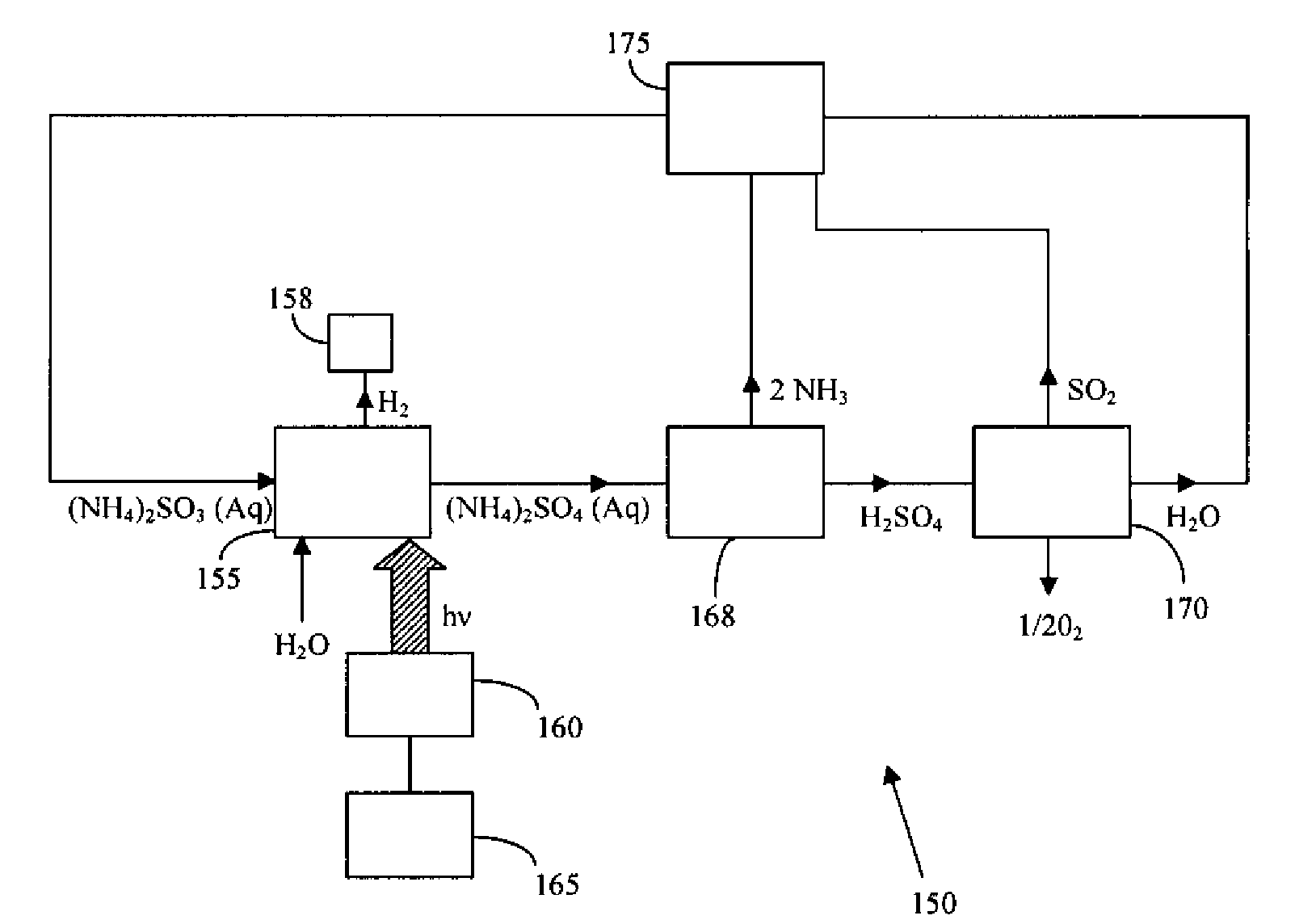
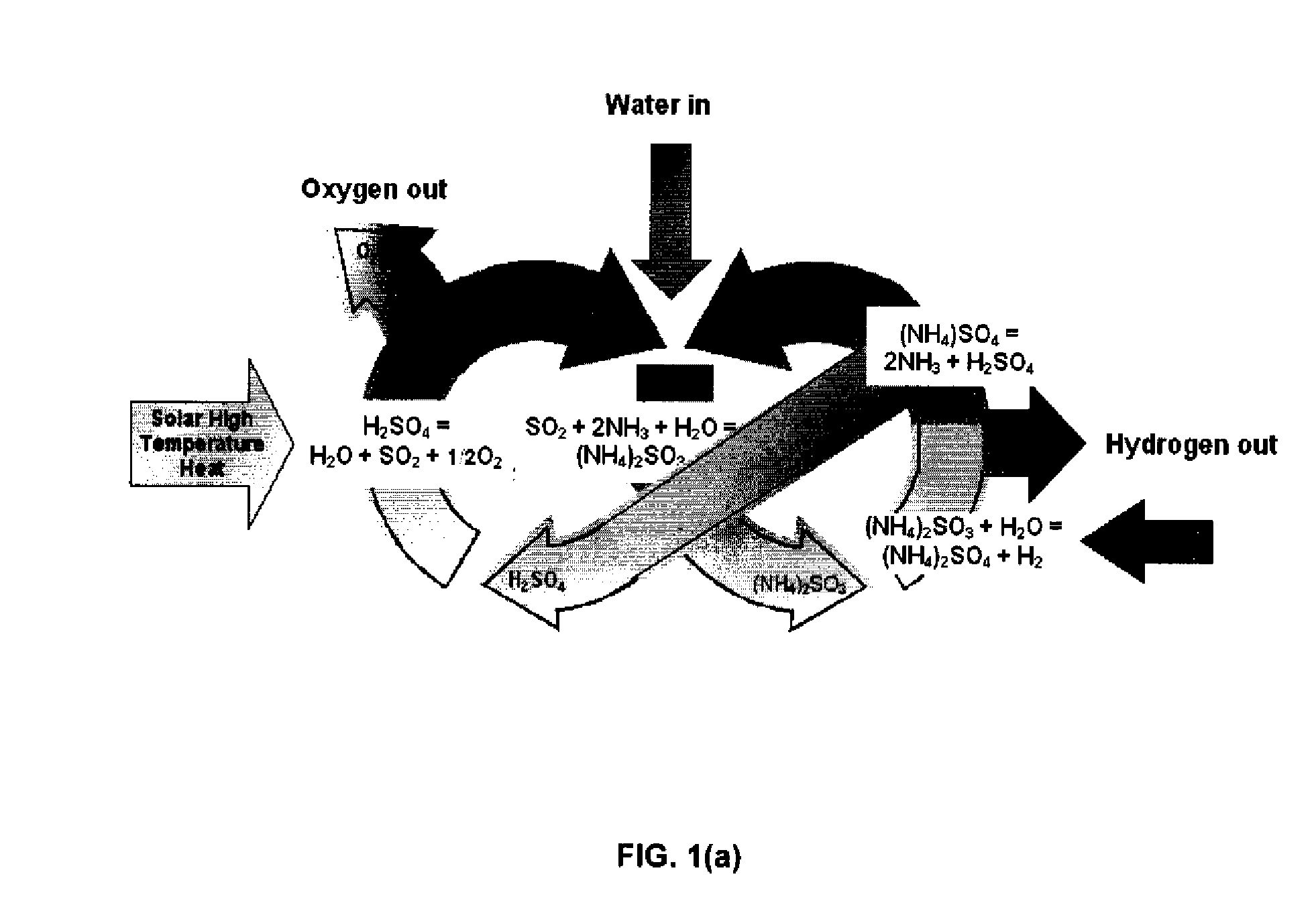
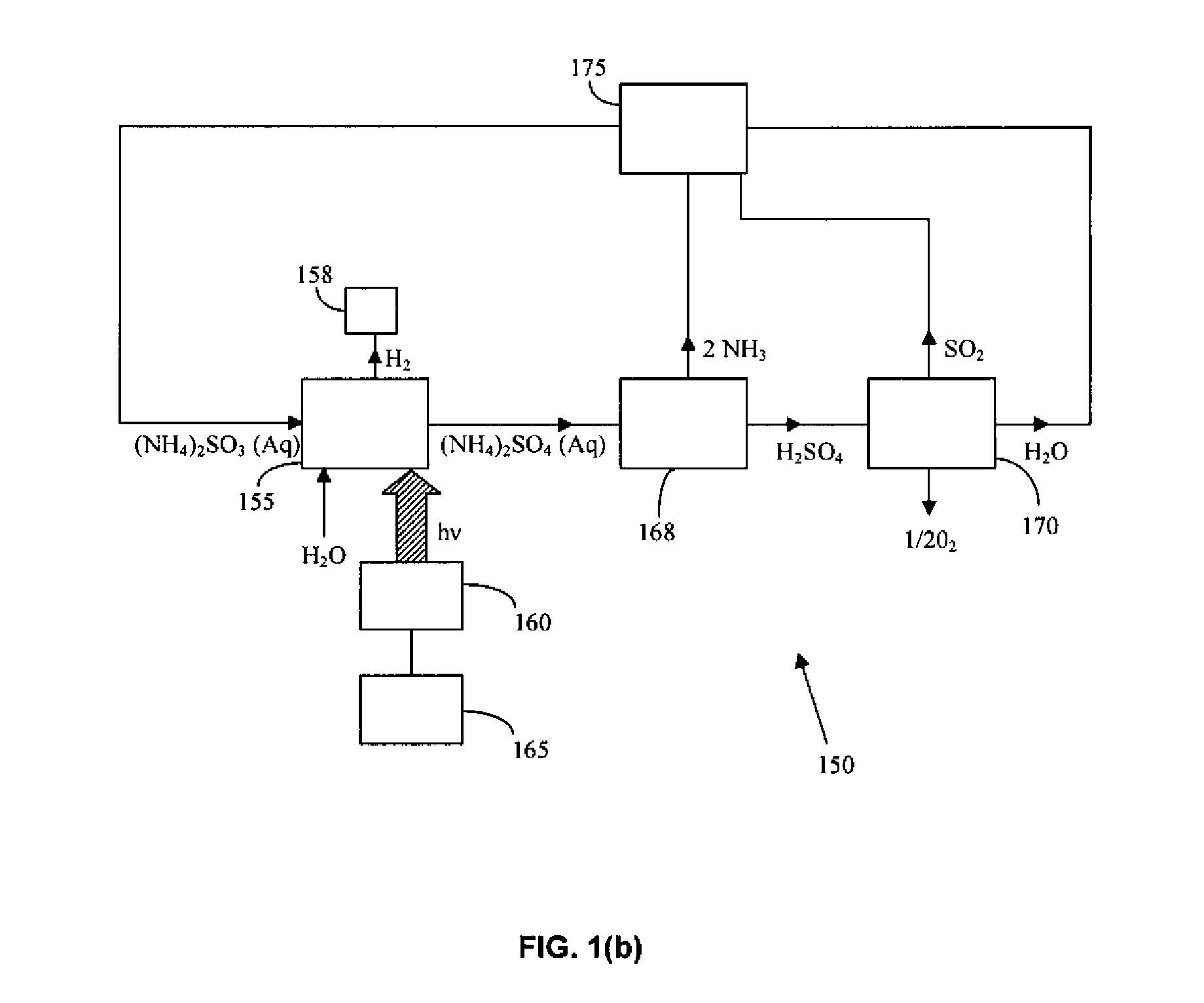
Thermochemical Cycle for Production of Hydrogen and/or Oxygen Via Water Splitting Processes
InactiveUS20080289951A1Hydrogen productionMagnesium/calcium/strontium/barium sulfides/polysulfidesWind drivenNuclear power
A method for the production of hydrogen via thermochemical water splitting includes the steps of providing an ammonium sulfite compound, dissolving the ammonium sulfite in water, and oxidizing the aqueous ammonium sulfite solution, wherein hydrogen is produced as a water reduction product associated with the oxidation. If purified air is used instead for the oxidation of aqueous ammonium sulfite solution, the method produces oxygen from the purified air. In a preferred embodiment of the invention, the oxidation is a photooxidation. Light for the photoxidation can be provide by a direct light source, such as solar energy, or indirectly from conversion of electrical energy to light, such as using a UV or visible light lamp. Electrical energy can be provided by a variety of sources, including low cost sources comprising wind driven, water driven (hydroelectric), or nuclear power.
Owner:HUANG CUNPING +2
Technique for producing magnesium hydroxide, barium choride and sulfureted hydrogen from brine water in salt lake
InactiveCN1415541AEase of mass productionSimple processCalcium/strontium/barium chloridesCalcium/strontium/barium sulfatesHydrogenBarium dichloride
A process for preapring magnesium hydroxide, barium chloride and hydrogen sulfide from the bittern of salt lake includes such steps as filtering, reaction on barium chloride to obtain raw bittern, thermal reaction on barium hydrosulfide to obtain hydrogen sulfide gas, filter, washing deposit with deionized water to obtain magnesium hydroxide, regulating pH value of filtrate, desulfurizing, neutralizing, depositing, and evaporating the supernatant to obtain barium chloride. Its advantages are high quality of products and low cost.
Owner:NAFINE CHEMICAL INDUSTRY GROUP CO LTD
Semiconductor ultrafine particles, fluorescent material, and light-emitting device
InactiveUS20070075294A1Increase brightnessImprove light resistanceMechanical apparatusPoint-like light sourceQuantum yieldLight equipment
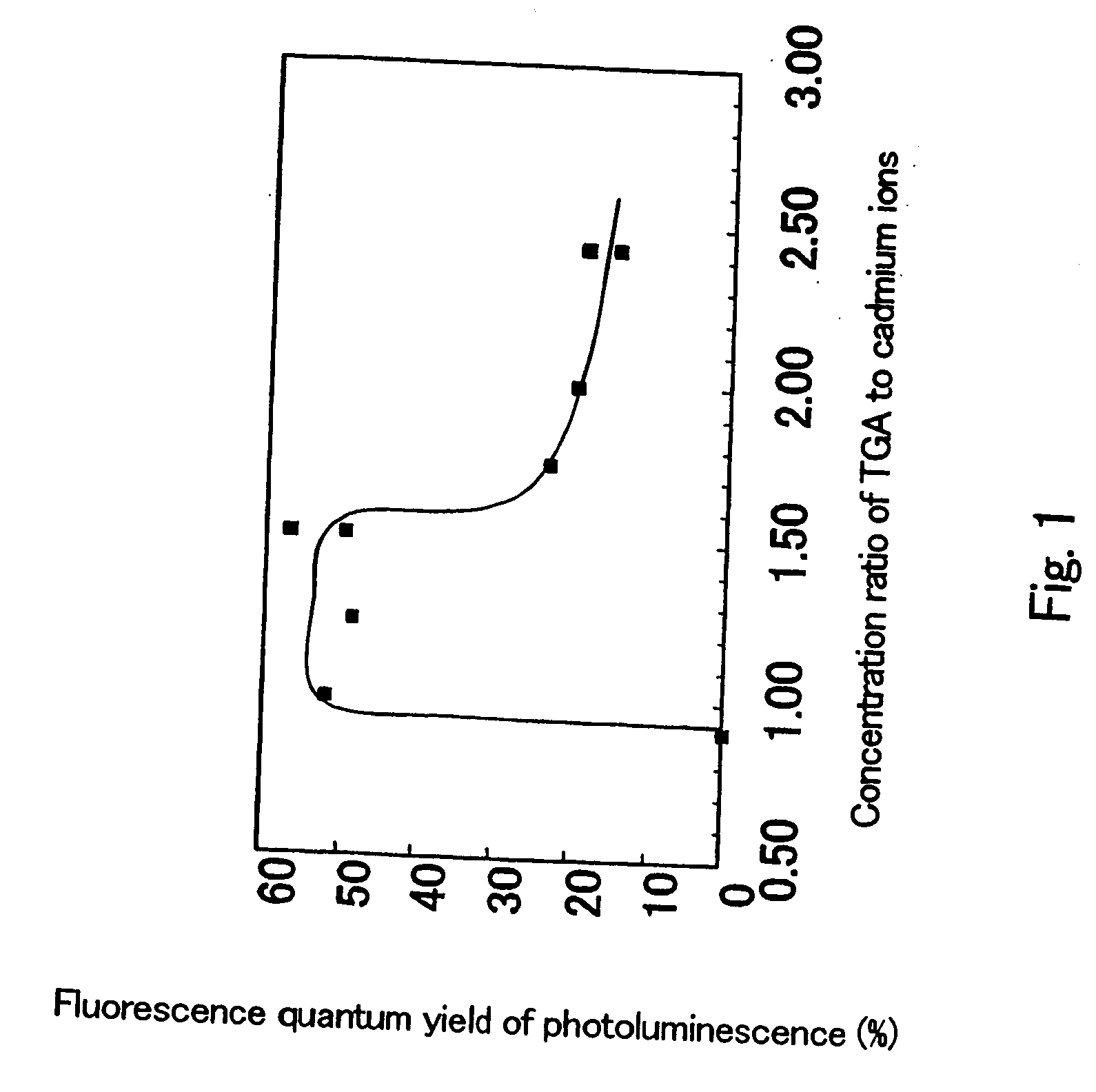
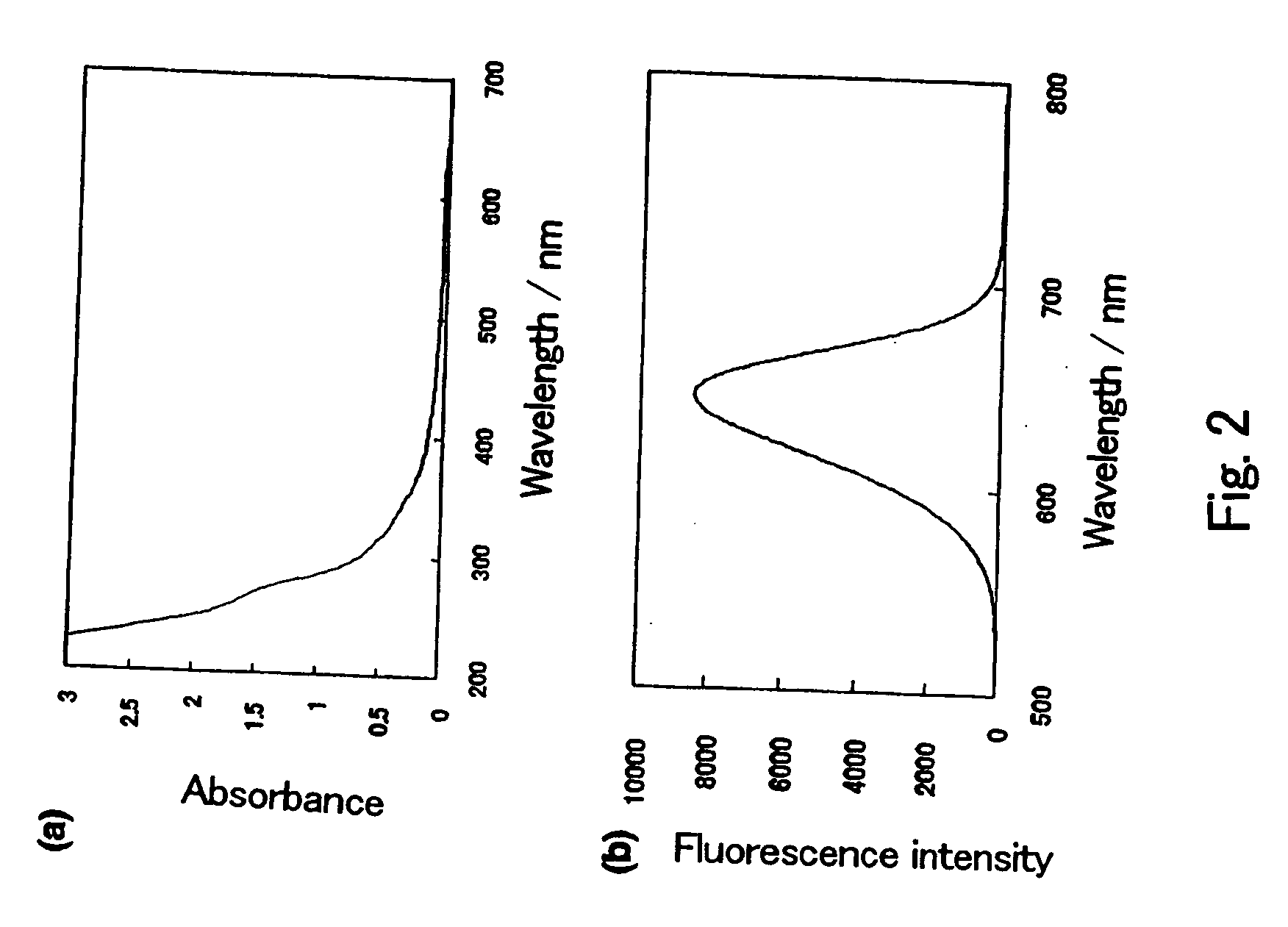
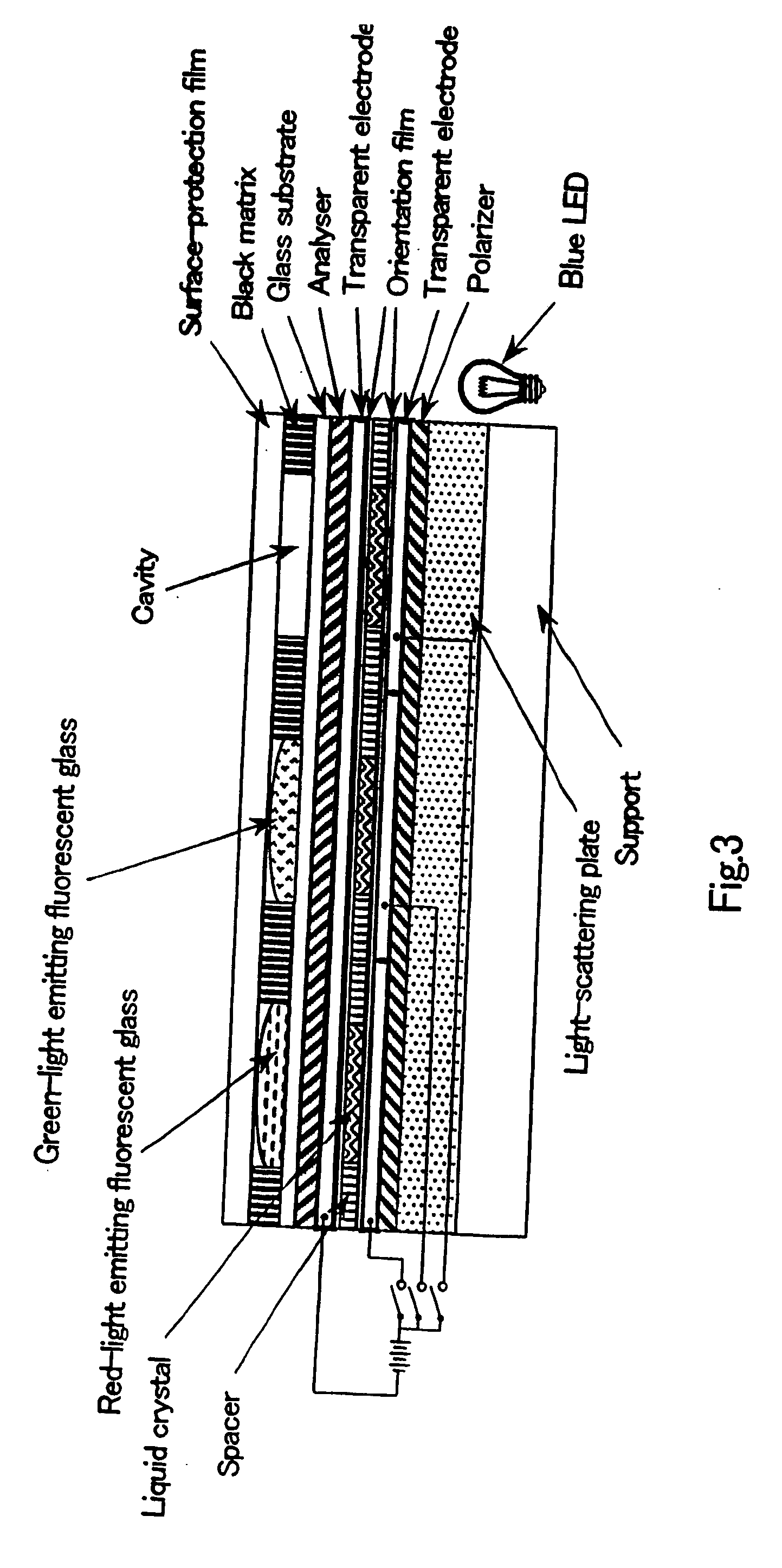
The present invention provides a novel fluorescent material which has a luminance higher than that of the conventional rare earth ion-dispersed fluorescent materials and is excellent in light resistance and long-term stability, and also an optical device, such as a high-luminance display panel or lighting equipment, which uses such a fluorescent material. Semiconductor ultrafine particles are characterized by maintaining 50% or more fluorescence quantum yield of photoluminescence when they are kept dispersed in water at 10° C. to 20° C. in air for 5 days. The fluorescent material is obtained by dispersing such semiconductor ultrafine particles in a glass matrix using a sol-gel process.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com