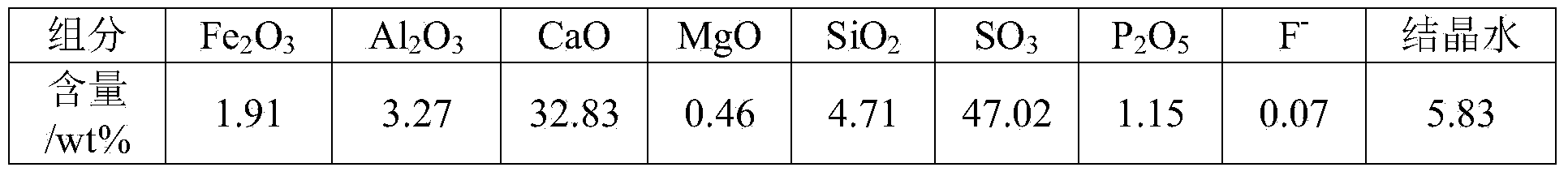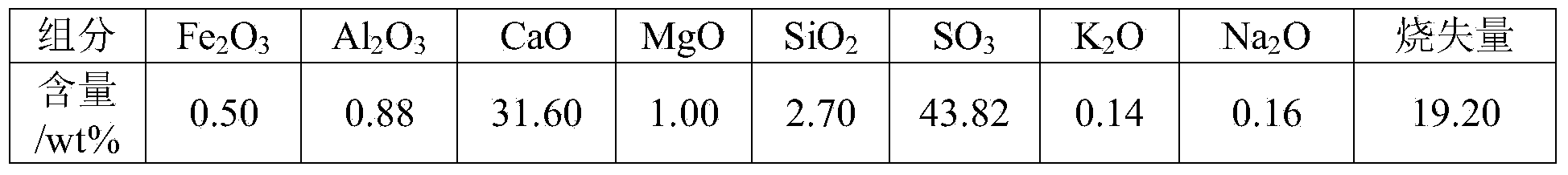Patents
Literature
243 results about "Calcium sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
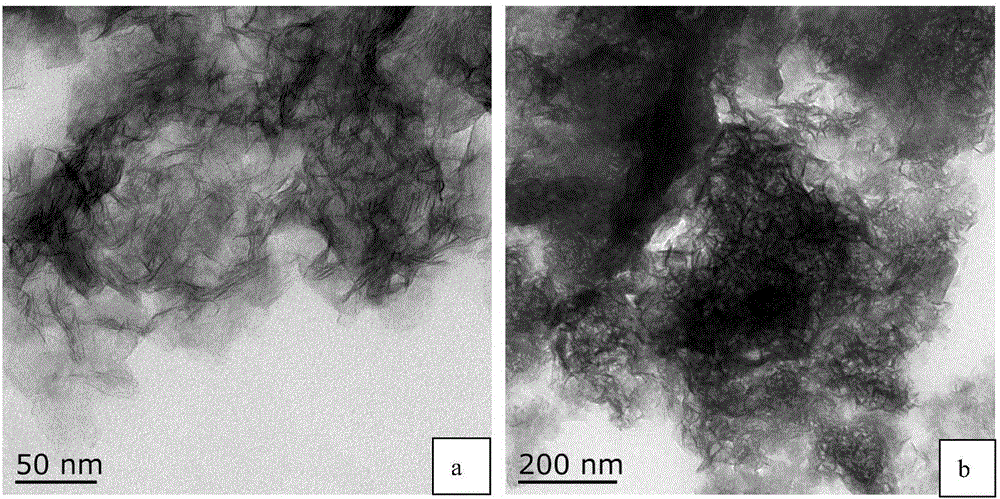
Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H₂S, which results from small amount of this gas formed by hydrolysis of the salt.
Sorbents for Removal of Mercury from Flue Gas
InactiveUS20070092418A1Enabling useLow costGas treatmentSolid waste managementParticulatesAlkaline earth metal
Metal sulfides having a micro-porous structure are disclosed for use as sorbents for removal of mercury from flue gas. Systems are disclosed for making and using micro-porous particulates at least partially composed of alkaline earth metal and transition metal sulfides as sorbents. Calcium sulfide micro-porous powders derived from the high temperature reduction of calcium sulfate and calcium sulfite are disclosed to be reactive substrates for a group of sorbents for adsorption of mercury from the myriad of coal combustion flue gases produced by the utilities industry, as well as from natural gas and gaseous and liquid hydrocarbons. Controlled addition of one or more of polyvalent metal ions, chloride ions, polysulfide ions, and sulfur to the micro-porous calcium sulfide substrate produces the sorbent. The sorbents are useful for cost-effectively adsorbing elemental mercury and oxidized mercury species such as mercuric chloride from flue gases, including those containing acid gases (e.g., SO.sub.2, NO and NO.sub.2, and HCI), over a wide range of temperatures.
Owner:CHEM PROD CORP
Method for reducing and decomposing phosphogypsum by sulfur
ActiveCN101708826AImprove recovery rateReduce reaction energy consumptionSulfur compoundsReduction rateSlag
The invention discloses a method for reducing and decomposing phosphogypsum by sulfur, which comprises the following steps: putting the phosphogypsum into a reactor, and raising the temperature to between 500 and 900DEGC for preheating for 10 to 30 minutes at an inert atmosphere; introducing gaseous sulfur in a molar percentage of 10 to 50 percent to perform reduction reaction with the phosphogypsum for 1 to 2 hours; grinding obtained a calcium sulfide block, and uniformly mixing the ground calcium sulfide with the phosphogypsum in a molar ratio of 1-1.5:3; sintering at a temperature between 1,000 and 1,400 DEG C for 0.5 to 3 hours at a non-oxidizing atmosphere; and taking CaO in the obtained solid slag as a cement clinker for cement production, taking the generated tail gas SO2 as a raw material gas for producing sulphuric acid. The reduction rate of CaSO4 is high, the decomposition ratio of the phosphogypsum can reach over 98 weight percent, the desulfurization ratio of the phosphogypsum can reach over 95 weight percent, and the method has the advantages of low energy consumption, simple and mature process, short production period, easy control and convenient promotion.
Owner:SICHUAN UNIV
Method for improving SO2 concentration in acid making technique with decomposition of calcium sulphate
ActiveCN101244811AIncrease concentrationStable productionSulfur-trioxide/sulfuric-acidVulcanizationSulfate
The invention relates to a method for improving the concentration of the SO2 in decomposing the calcium sulfate to acid craft and a chemical manufacturing technique of sulphuric acid and cement clinker, in particular to a manufacturing technique of sulphuric acid and cement clinker made from calcium sulfate. The procedures of the invention are as follows: a, under the function of the strong reduction medium, the calcium sulfate is produced into calcium vulcanization in the reactor; b, the reduction product of a procedure is the compound of the calcium vulcanization and the unreacted calcium sulfate; c, the reduction product is transferred to another reaction directly and under the high temperature, the calcium vulcanization and the calcium sulfate react and make into the SO2 and the clinker. The method has the advantages that: the concentration of the SO2 in gas is increased; the production system of sulfate is more stable and the extensive use of phosphogypsum is possible.
Owner:YUNNAN YUNTIANHUA
Sorbents for Removal of Mercury From Flue Gas Cross Reference To Related Applications
Systems are disclosed for making and using micro-porous particulates at least partially composed of metal sulfides, particularly alkaline earth metal and transition metal sulfides, as sorbents for removal of mercury from flue gas. Calcium sulfide micro-porous powders derived from the high temperature reduction of calcium sulfate and calcium sulfite are disclosed to be reactive substrates for a group of sorbents for adsorption of mercury from coal combustion flue gases produced by the utilities industry, as well as from natural gas and gaseous and liquid hydrocarbons. The sorbents are useful for cost-effectively adsorbing elemental mercury and oxidized mercury species such as mercuric chloride from flue gases, including those containing acid gases (e.g., SO.sub.2, NO and NO.sub.2, and HCl), over a wide range of temperatures.
Owner:CHEM PROD CORP
Lubricating oil compsn.
InactiveCN1415712AMeet specificationsFulfil requirementsLubricant compositionFoaming agentCalcium sulfide
A composite lubricaitng oil conforming with the specification of GB 11121 and API SF / CD universal lubricating oil of IC engine contains neutral basic oil, magnesium naphthenate as cleaning agent, alkylphenol calcium sulfide, polyisobutylene butanediimide, zinc dialkyldithiophosphate, auxiliary antioxidizing agent, viscosity index improver, pour depressor, and anti-foaming agent.
Owner:PETROCHINA CO LTD
Method for utilizing comprehensive resource of sulfur-containing solid waste
InactiveCN101570341ATo promote metabolismSave resourcesDispersed particle separationSulfur compoundsSulfur containingPollution
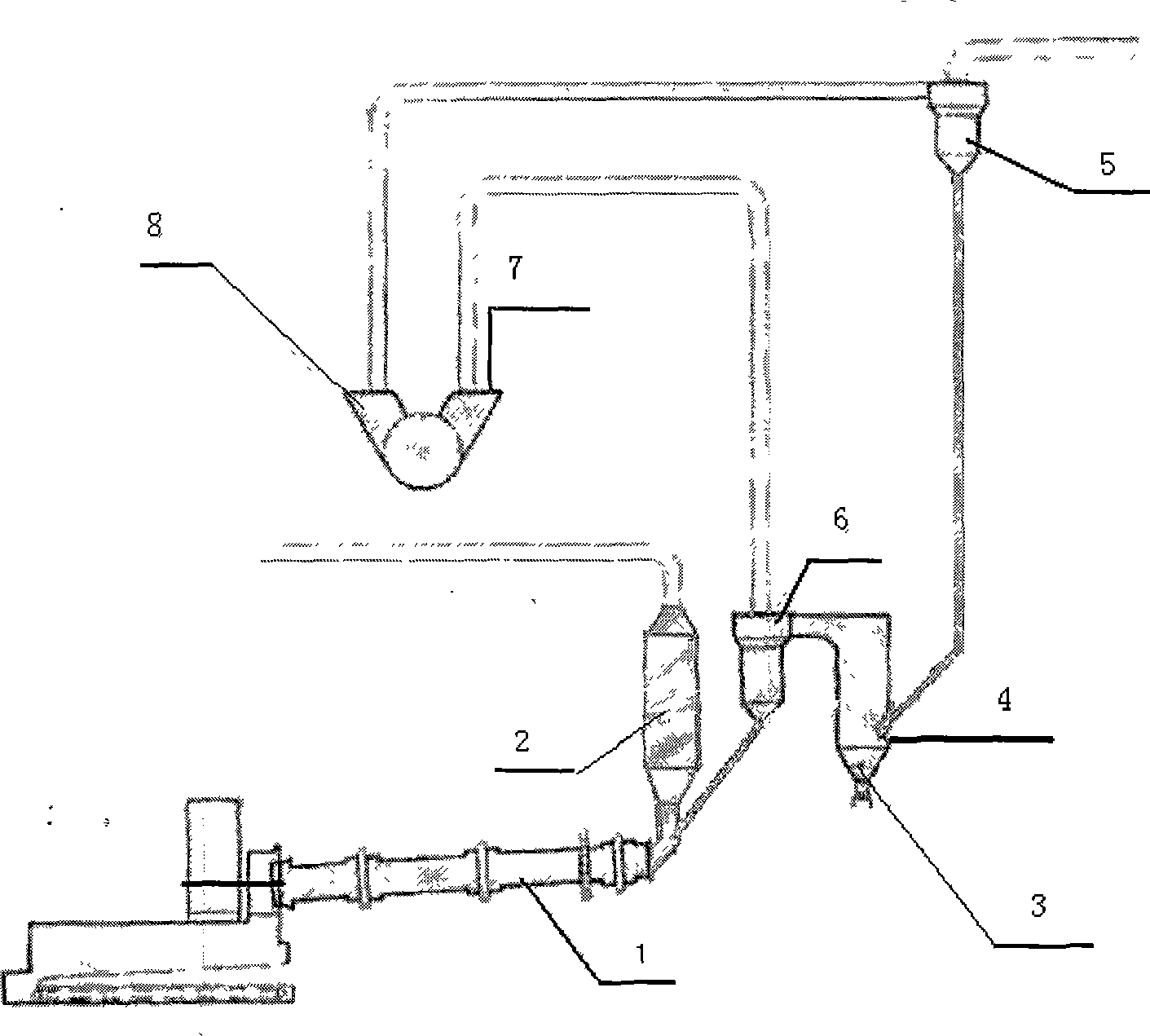
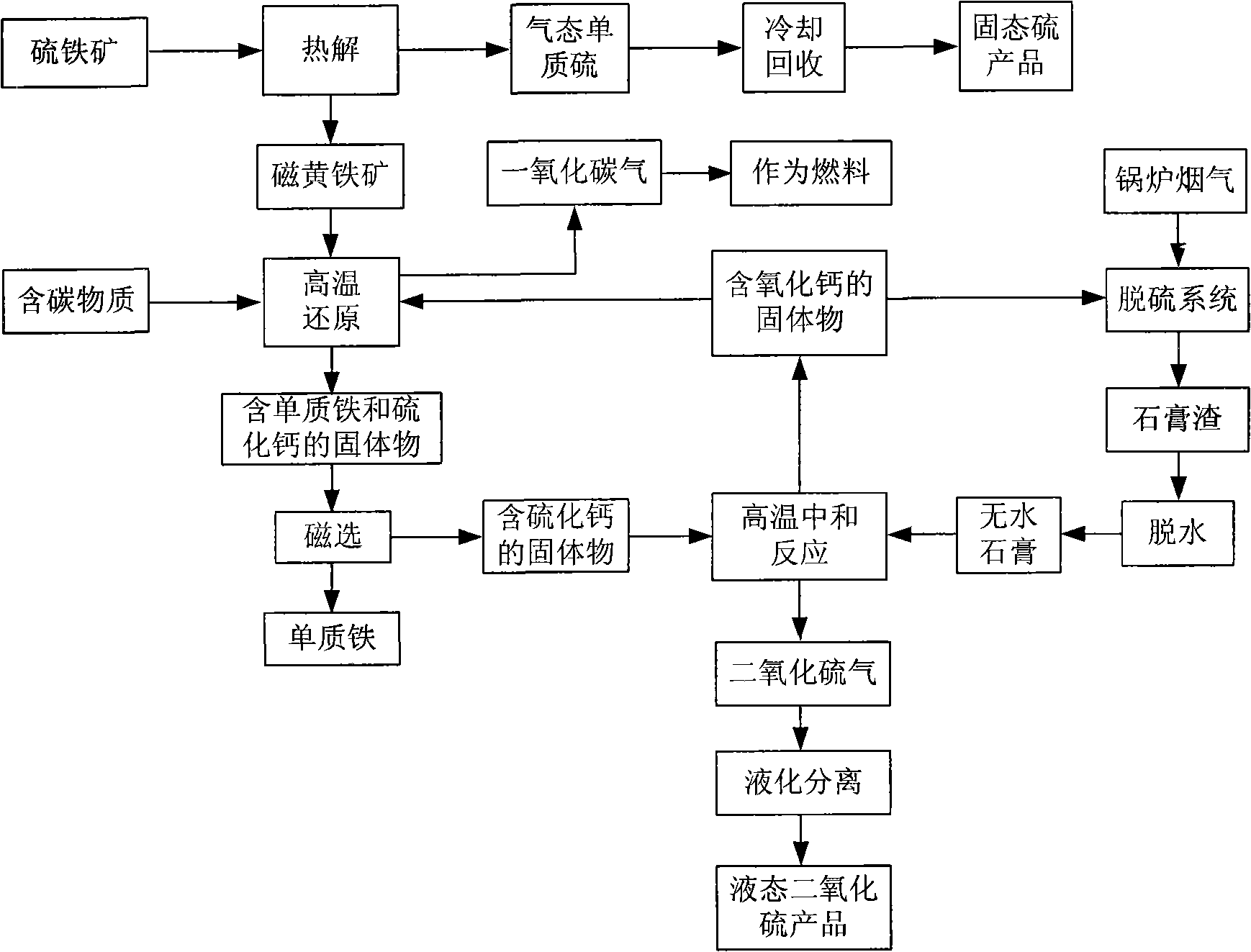
The invention discloses a method for utilizing comprehensive resource of sulfur-containing solid waste, which comprises the following steps: (1) pyrolyzing sulfurous iron ore to obtain pyrrhotite and an elemental sulfur product; (2) mixing the pyrrhotite, a carbon-containing substance and a calcium-containing substance to carry out reduction reaction under a high temperature to obtain a solid mixture of elemental iron and calcium sulfide; (3) magnetically separating the solid mixture, and separating and recovering the solid mixture to obtain the elemental iron; (4) mixing and heating the solid containing the calcium sulfide and dehydrated gypsum to obtain tail gas containing SO2 and solid containing calcium oxide; and (5) separating the tail gas containing the SO2 through liquefying to obtain a liquid SO2 product, and using the solid containing the calcium oxide as the calcium-containing substance to participate in the reaction of the step (2) and as flue gas desulfurizing agent for cyclically utilization. The method solves the problems of occupied floor due to the stacking of desulfurization gypsum and coal washing sulfurous iron ore, environment pollution and potential safety hazard, makes full use of calcium, sulfur and iron resources rich in the wastes, and can realize recycling economy and clean production.
Owner:HUAZHONG UNIV OF SCI & TECH
Method for producing sulfur dioxide from calcium sulfate and sulfur
ActiveCN102502524AQuick responseImprove heat utilizationChemical industrySulfur compoundsClinker (cement)Sulfur dioxide
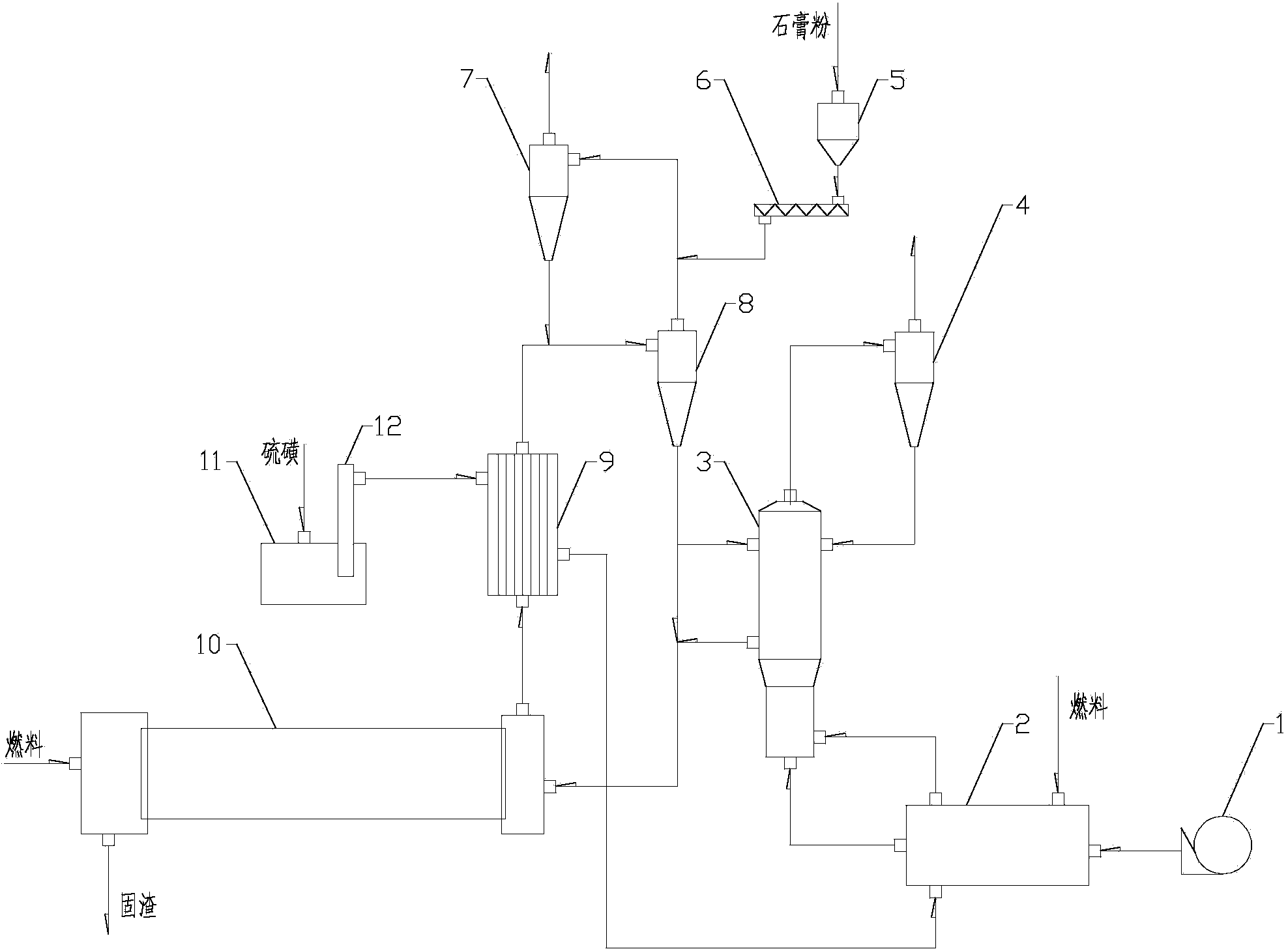
The invention provides a method for producing sulfur dioxide from calcium sulfate and sulfur and relates to a chemical production process for producing sulfuric acid and cement clinker by adopting calcium sulfate. The method comprises the following steps: a, drying wet calcium sulfate into semi-hydrated gypsum, mixing other correction raw materials into the semi-hydrated gypsum to prepare a raw material, adding the raw material into a material preheating system consisting of multiple stages of cyclone preheaters, preheating, and then feeding into a reduction furnace; b, spraying liquid sulfur into the reduction furnace; c, controlling the content of oxygen in the reduction furnace to be less than 1.5%, and reducing calcium sulfate to generate calcium sulfide; and d, controlling the molar ratio of calcium sulfide generated in the step c to unreacted calcium sulfate to be (1:2)-(9:1), feeding a mixture of calcium sulfide and unreacted calcium sulfate into a rotary kiln and then reacting at oxidation atmosphere at the temperature of 900-1450 DEG C so as to obtain SO2 and the cement clinker. The method is rapid in reaction speed (the reaction time is only few seconds under fluidization), high in heat utilization rate, simple in process, and easily controllable in reaction condition.
Owner:YUNNAN YUNTIANHUA
Special lubricating oil for fuel gas engine of public transport automobile
ActiveCN104342266AAntioxidant time is longImprove protectionLubricant compositionThiadiazolesPolyisobutylene succinimide
The invention discloses special lubricating oil for a fuel gas engine of a public transport automobile. The special lubricating oil is prepared from blended base oil, binary ethylene-propylene rubber, polymethacrylate, alkylphenol calcium sulfide, high-base-number calcium alkylbenzenesulfonate, polyisobutylene succinimide, boronated polyisobutylene succinimide, alkylated phenyl-alpha-naphthylamine, p,p'-dioctyl diphenylamine, a hindered phenol type antioxidant, dialkyl molybdenum dithiocarbamate, copper nanoparticle anti-wear additives, methyl silicone oil and thiadiazole polysulfide. According to the lubricating oil composition oil product, the sulfate ash content is lower than 0.5%, the content of phosphorus is lower than 0.03%, and the base number is 3-8mgKOH / g. According to the special lubricating oil for the fuel gas engine of the public transport automobile, aiming at the special requirements of the public transport automobile on engine oil, different additive varieties and charging sequences are adopted, so that physical and chemical indicators and use performance of the special lubricating oil are better than natural gas engine lubricating oil of the same grade, and the special lubricating oil has excellent cleaning and dispersion properties, lubricating property, abrasion resistance and oxidation resistance.
Owner:陕西通用润滑科技有限公司
Method for preparing red luminescence material of calcium sulfide or alkaline earth sulphides activated by rare earth europium
InactiveCN1916109AEasy to operateReduce manufacturing costLuminescent compositionsAlkaline earth metalRare earth
This invention relates to a method for preparing rare earth Eu-activated calcium sulfide or alkaline earth metal sulfide red-fluorescent material. The method comprises: (1) weighing calcium carbonate or alkaline earth metal carbonate, europium oxide and sulfur according to the chemical formula of Ca1-xSn : Eux or MyCa1-x-ySn : Eux (x = 0.001-0.04 mol, n = 1-3, M = one or more of Mg, Sr, Ba and Zn, and y = 0-1); (2) grinding and mixing the raw materials completely, and placing in a torrefaction container; (3) torrefying at 900-1200 deg.C for 30-120 min to obtain red-fluorescent powder. The method has such advantages as simple operation, low cost and no harm to operators. The emitting peak of the fluorescent material can change within the range of 50 nm, thus is suitable for different circumstances.
Owner:上海宝鼎机械设备有限公司
Method for absorbing sulfur dioxide and co-producing sulphur
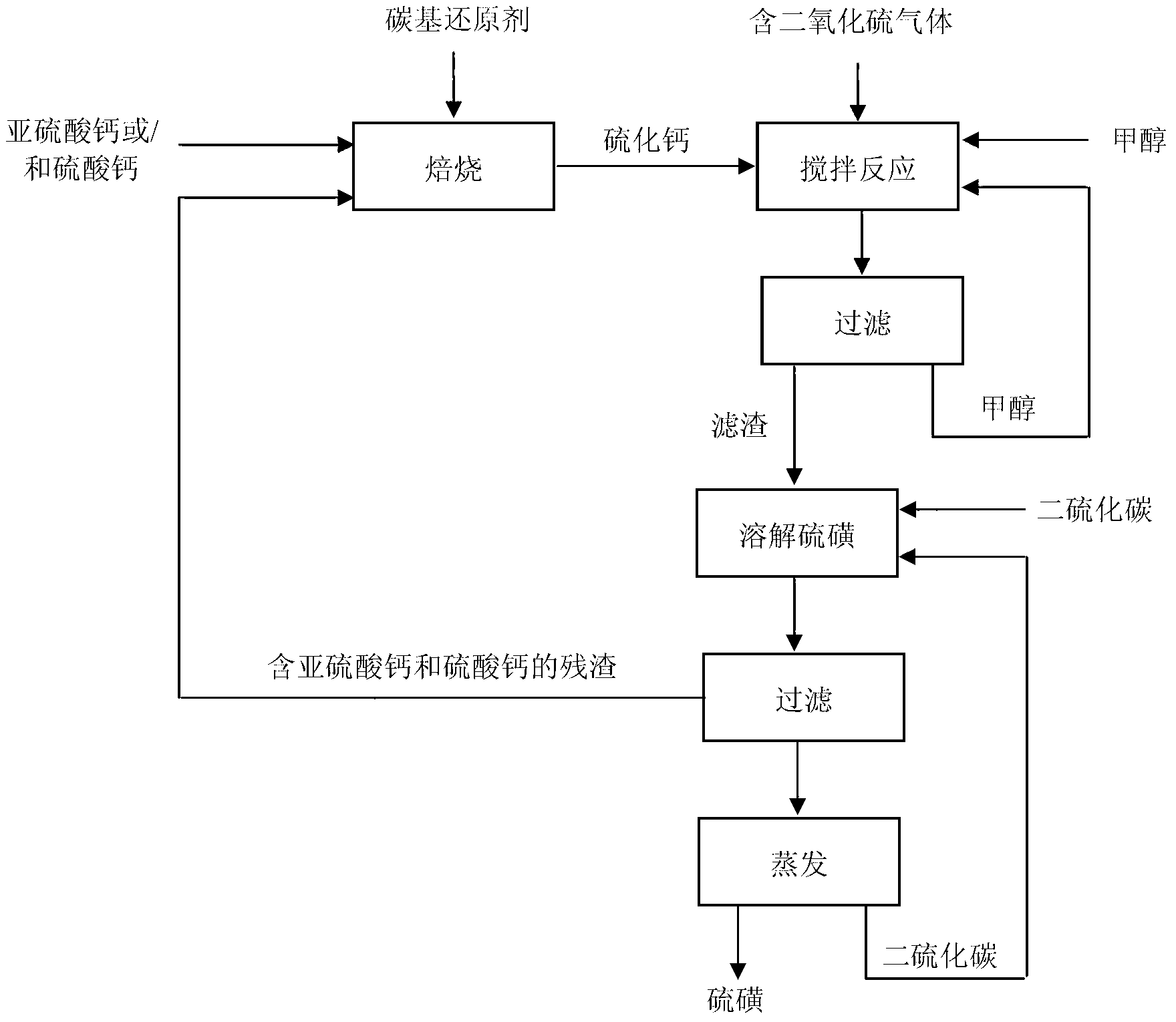
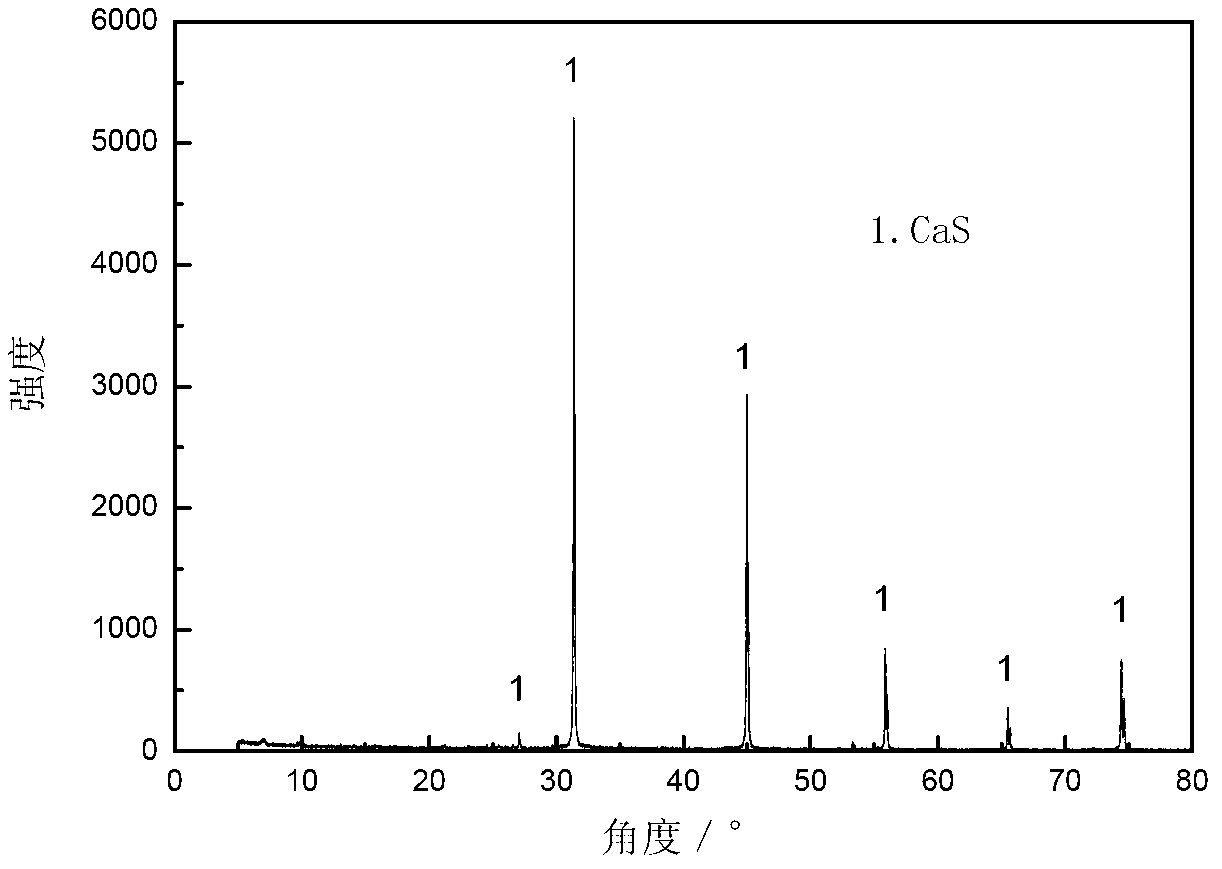
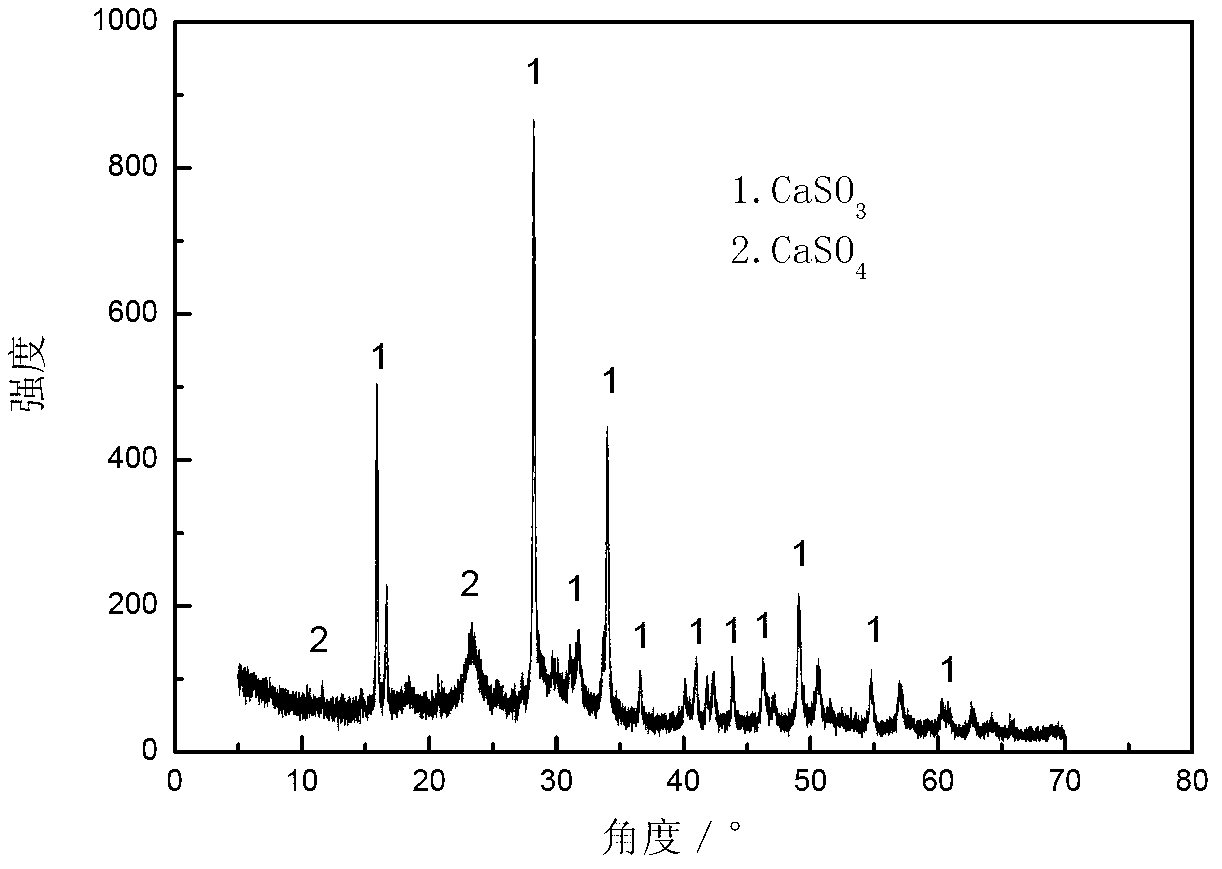
ActiveCN103316578ASubstantiveEffective absorptionDispersed particle separationSulfur preparation/purificationCalcium sulfideSulfate
The invention provides a method for absorbing sulfur dioxide and co-producing sulphur. The method comprises the following basic steps of: mixing calcium sulfite or / and calcium sulfate with a carbon-based reducing agent; roasting for 1.5 to 2 hours at 800 to 1,000 DEG C to obtain calcium sulphide; adding calcium sulphide, methanol or / and ethanol to a reactor; adjusting the liquid-solid mass ratio to 3 to 10:1; charging sulfur dioxide containing gas along with stirring; stirring for reacting sulfur dioxide with calcium sulphide under room temperature of 0 to 40 DEG C; separating after the reaction is done to obtain sulphur and a mixture containing calcium sulfite and calcium sulfate. By adopting the method, the sulfur dioxide in the gas can be absorbed, and the sulfur can be recovered; the method has the advantages of being economic, efficient and free of waste residues.
Owner:CENT SOUTH UNIV
Sorbents for removal of mercury from flue gas cross reference to related applications
InactiveUS7771700B2Solid waste managementUsing liquid separation agentParticulatesAlkaline earth metal
Owner:CHEM PROD CORP
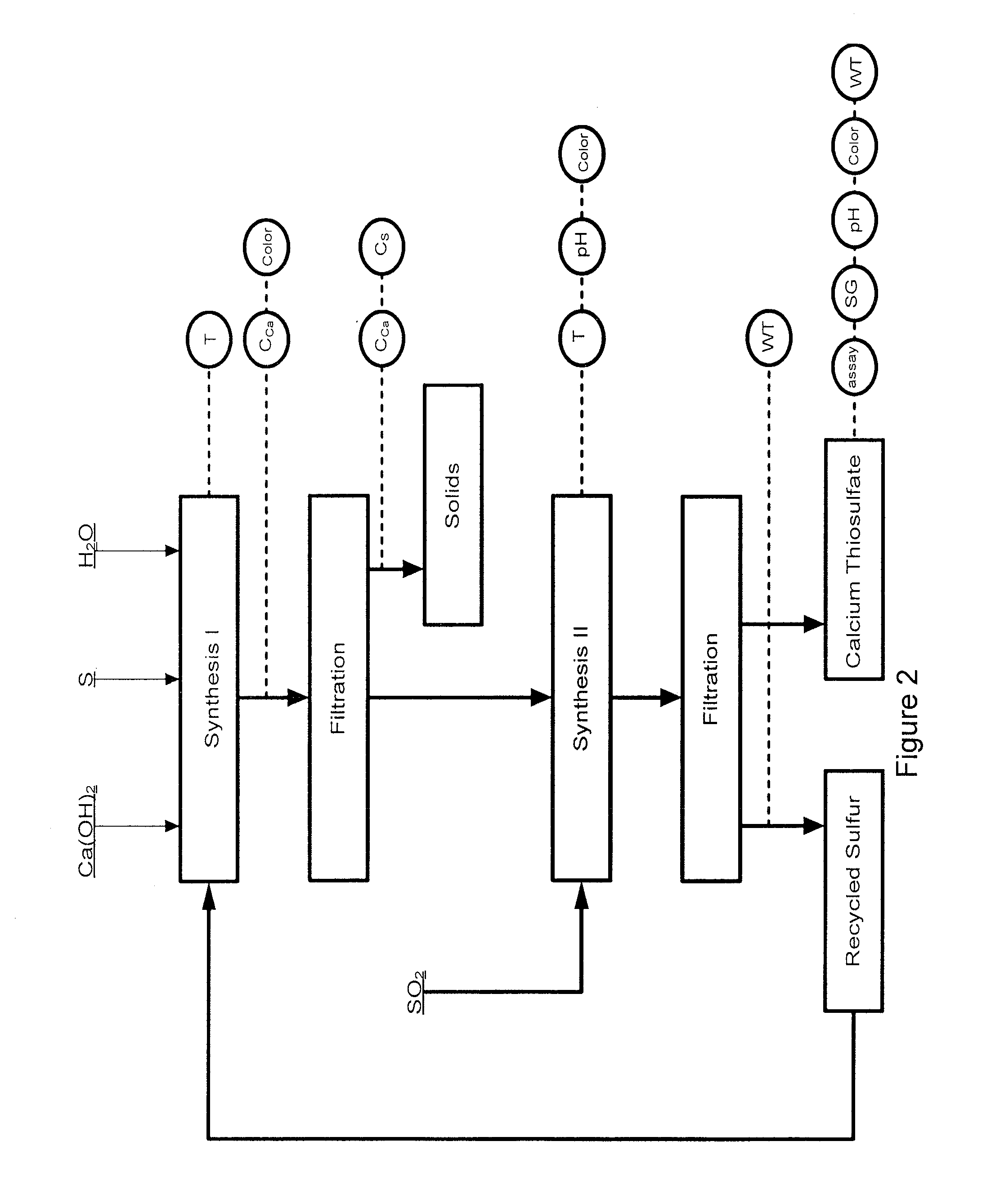
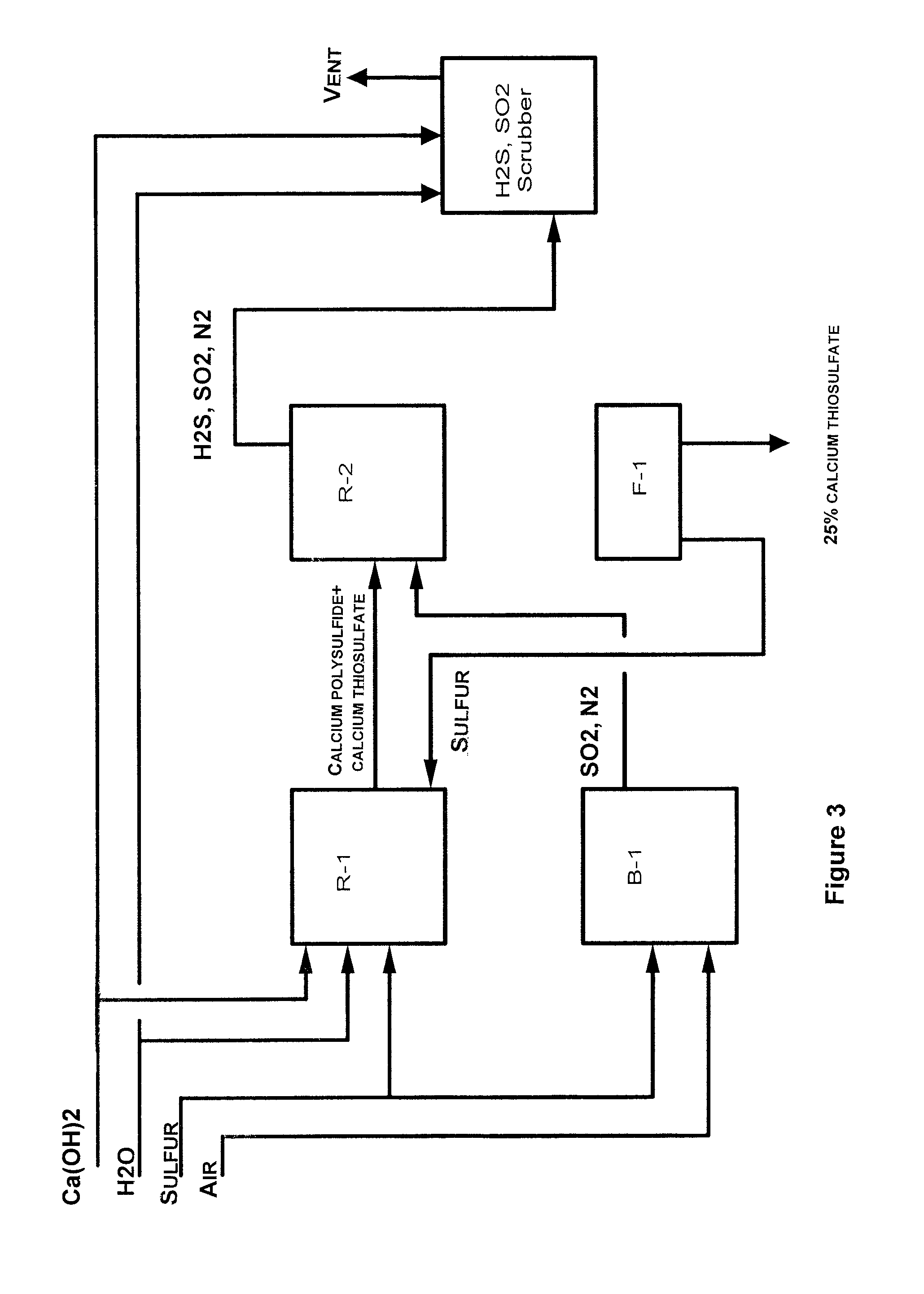
Process for preparation of calcium thiosulfate liquid solution from lime, sulfur, and sulfur dioxide
ActiveUS8034318B1Reduces formation of undesirableIncrease concentrationThiosulfates/dithionites/polythionitesDispersed particle separationHigh concentrationEmulsion
An efficient batch or semi-continuous method of production of relative high concentration, low byproduct-containing, calcium thiosulfate (CaS2O3) from lime, sulfur or calcium polysulfide, and sulfur dioxide under elevated reaction temperature conditions is described. The product is an emulsion of liquid calcium thiosulfate and solid byproducts. Under the conditions of the art, including the mole ratios of lime to sulfur, the temperature of the reaction process and the sulfur dioxide reaction conditions, including rate and duration, the byproducts are reduced to about 2% by weight.
Owner:TESSENDERLO KERLEY INC
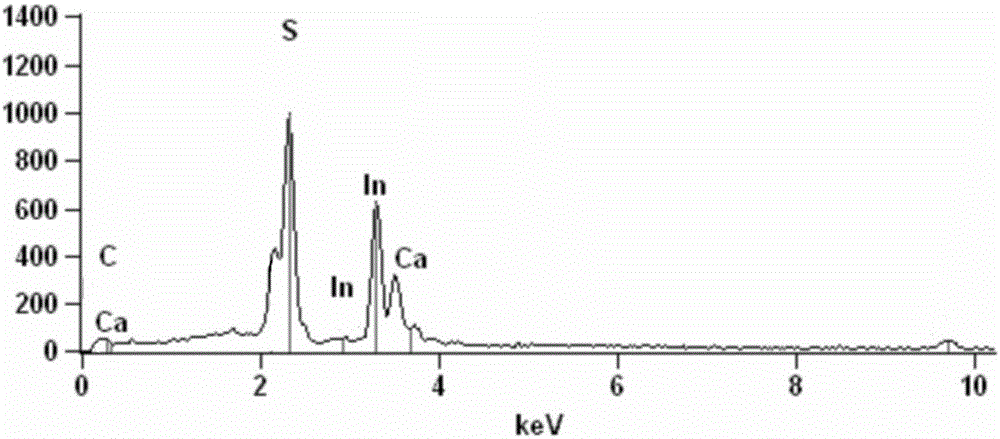
Method for preparing carbon quantum dot/flower-shaped indium and calcium sulfide composite photocatalysts and application thereof
ActiveCN106268869ANo emissionsLow costPhysical/chemical process catalystsWater/sewage treatment by irradiationIndiumTetracycline Hydrochloride
The invention discloses a method for preparing novel carbon quantum dot / flower-shaped indium and calcium sulfide (CQDs / CaIn2S4) composite photocatalysts, and belongs to the field of environmental protection. The method includes preparing the CQDs / CaIn2S4 composite photocatalysts by the aid of in-situ hydrothermal processes at certain temperatures. The CQDs / CaIn2S4 composite photocatalysts prepared by the aid of the method can be applied to catalytically degrading tetracycline hydrochloride solution under the sunlight or visible light. The method has the advantages that the method includes simple technologies, is free of pollutant discharge in preparation procedures and is short in preparation period and low in energy consumption and cost, is a green synthesis technique, and the carbon quantum dot / flower-shaped indium and calcium sulfide composite photocatalysts can be prepared on a large scale; the visible light response and the adsorption capacity of the carbon quantum dot / flower-shaped indium and calcium sulfide composite photocatalysts can be improved after CQDs (carbon quantum dots) are compounded, the service lives of electrons-holes can be prolonged, photoelectron transmission can be promoted, and the visible light photocatalytic activity of the carbon quantum dot / flower-shaped indium and calcium sulfide composite photocatalysts can be greatly improved.
Owner:JIANGSU UNIV
Method for preparing calcium sulfide by reducing and decomposting gypsum through sulfur
InactiveCN101708825AImprove recovery rateAvoid Oxidation DisadvantagesMagnesium/calcium/strontium/barium sulfides/polysulfidesReduction rateCalcium sulfide
The invention discloses a method for preparing calcium sulfide by reducing and decomposting gypsum through sulfur, which is characterized in that: the method comprises the following steps: firstly, putting the gypsum into a reactor and raising the temperature to between 500 and 900DEG C for preheating 10 to 30 minutes at an inert atmosphere; and then introducing gaseous sulfur to perform reduction reaction with the gypsum for 0.5 to 2 hours to obtain a block, namely a calcium sulfide product, wherein the generated tail gas SO2 is used for producing sulphuric acid, and the gaseous sulfur accounts for 10 to 50 mol percent of mixed atmosphere in a reaction system. Because the method adopts a gas-solid reaction as a reaction mechanism, and the reaction is carried out at the inert atmosphere, the method has the advantages of low energy consumption, high reduction rate of over 95 percent, simple and mature process, short production period, easy control and convenient promotion.
Owner:SICHUAN UNIV
Agent and method for harmless gelation treatment of well cuttings
InactiveCN104628324AHigh Consolidation Removal AbilityReduce orderSolid waste managementSodium phosphatesHazardous substance
The invention discloses an agent for harmless gelation treatment of well cuttings. The agent comprises the following raw materials in parts by weight: silicate, cement, aluminum salt, additives, calcium sulfide, a strength accelerator, a plasticizer, sodium phosphate, sodium sulfide and a stability-breaking gel. The agent for gelation treatment has the advantages of short curing period, high consolidation strength, high consolidation elimination capability to harmful substances, and high washing and soaking resistance. The agent can be used for curing the well cuttings relatively low in moisture content, and also can be used for curing in a water soaking environment in which the cuttings are extremely high in moisture content to achieve the same good effect; the time of waiting on cement setting is extremely short; people can walk on the cured cuttings 18 hours later after curing; the cured cuttings are capable of having the cement brick hardness after being placed for about 3 days, and capable of having the clay brick hardness in about 6 days. The cured well cuttings can be applied to the backfill construction of a well site, and therefore, the earthwork cost can be saved, furthermore, the hidden danger of pollution accidents is eliminated, the oilfield ecologic environment is protected, and the investment is also saved. The agent has good economic benefit and environment benefit.
Owner:四川吉奥安欣环保科技有限公司 +2
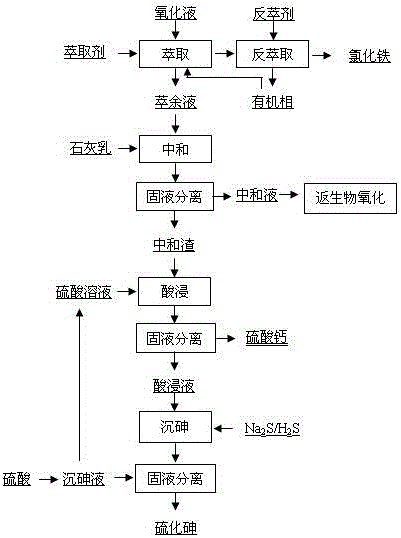
Method for recycling valuable elements in acidic biological oxidation solution containing arsenic, iron and sulfur
ActiveCN106521162AEasy to storeAchieve recyclingProcess efficiency improvementHydrometallurgyArsenic sulfide
The invention relates to a method for recycling valuable elements in an acidic biological oxidation solution containing arsenic, iron and sulfur and belongs to the field of hydrometallurgy. The method comprises the following steps: performing three-stage counter-current extraction on the oxidation solution and an extracting agent so that iron ions in the oxidation solution can turn into an organic phase so as to be separated and recycled; adding a precipitator to the iron-removed raffinate, and concentrating the elements such as arsenic, sulfur and a trace amount of copper, lead, zinc and iron in neutral dregs; leaching the ions of arsenic, copper, lead, zinc, iron and the like from the neutral dregs obtained after solid and liquid separation by using a sulfuric acid solution, and then performing solid and liquid separation to obtain a calcium sulfide product; adding sodium sulfide to a sulfuric acid leaching solution, precipitating arsenic ions in the solution in the form of arsenic sulfide and recycling arsenic in the form of a arsenic sulfide product after solid and liquid separation; and adding sulfuric acid to the arsenic-precipitated acidic solution, and repeating the step of leaching the arsenic in the neutral dregs. The method has the advantages that the used raw materials are easy to get and low in price; the technological process is simple and practical; comprehensive recovery rate of the valuable elements is high; and zero emission of wastes is realized.
Owner:CHANGCHUN GOLD RES INST
System and method for co-production of calcium oxide and sulphur through coal gasification synergistic with gypsum calcination
ActiveCN109809456ACalcium/strontium/barium sulfatesSulfur preparation/purificationHigh concentrationCalcination
The invention relates to a technology and method for co-production of calcium oxide and sulphur through coal gasification synergistic with gypsum calcination. Calcium sulfate and a reducing substancereact at the high temperature to generate calcium sulfide, the calcium sulfide can continue reacting with the calcium sulfate to generate calcium oxide and high-concentration SO2 flue gas, meanwhile,the calcium oxide can also react with the high-concentration SO2 flue gas at the low temperature to generate elemental sulfur steam, a U-shaped coal gasification calcining furnace, a gypsum second-stage calcining furnace, a reduction tower, a sulphur recovering device, various heat exchangers and the like are utilized, all reaction conditions are precisely controlled, thus the sulphur is preparedthrough low-grade gypsum difficult to treat, meanwhile, the calcium oxide is by-produced, and a by-product, namely the calcium oxide, can replace limestone to serve as a desulfurization and denitrification agent to be recycled.
Owner:SHANDONG UNIV
Method for producing light calcium carbonate and coproducing hydrogen sulfide by using crude calcium sulfide
InactiveCN101538060AReduce whitenessHigh solid impurity contentCalcium/strontium/barium carbonatesHydrogen sulfidesCALCIUM HYDROXIDE SOLUTIONCalcium sulfide
The invention relates to a method for producing light calcium carbonate and coproducing hydrogen sulfide by using crude calcium sulfide, belonging to the technical field of chemical production. The method comprises the steps of: pulverizing the crude calcium sulfide, and dissolving out the pulverized crude calcium sulfide by using saturated hydrogen sulfide solution at room temperature to obtain sulfur calcium hydroxide solution; removing other impurities in the crude calcium sulfide by filtering and washing, and introducing CO2 into clear solution to generate precipitation of light calcium carbonate and continuously generate hydrogen sulfide gas; preparing the saturated hydrogen sulfide solution by using part of the generated hydrogen sulfide gas, and delivering the rest of the hydrogen sulfide gas to a gas holder for storage; filtering and washing feed liquid generated from carbonatation reaction to obtain light calcium carbonate solid; introducing the hydrogen sulfide gas into the filtrate to prepare the saturated hydrogen sulfide solution, and returning to the previous step to dissolving out the crude calcium sulfide for recycling; and drying, crashing and packaging the light calcium carbonate to obtain a finished product of light calcium carbonate. The light calcium carbonate product has less impurity and high whiteness compared with that produced by the traditional method, and the byproduct hydrogen sulfide can be used as raw material of other chemical products.
Owner:GUIZHOU XIYANG FERTILIZER IND
Coal-burning installation based on calcium sulphate oxygen carrier and coal burning method
InactiveCN101012929AFluidized bed combustionApparatus for fluidised bed combustionFluidized bedCalcium sulfide
A coal burner based on calcium sulfate oxygen carrier and relative burning method realizes circulated oxygen carrier and the separation of carbon dioxide. The inventive device is formed by a circular fluid bed, a cyclone separator and a fluid bed into a circular loop. And the method comprises that oxidizing the calcium sulfide with air in the circular fluid bed to generate calcium sulfate, and to be separated by the cyclone separator; then the calcium sulfate is fed into the fluid bed; the coal of fluid bed is reacted with carbon monoxide which can reduce the calcium sulfate to generate the carbon dioxide and calcium sulfide, and the carbon dioxide is discharged via the air outlet of fluid bed; and part of carbon dioxide is extracted and circulated into the fluid bed; the reduced calcium sulfide is feedback to the circular fluid bed to be oxidized with the air. The invention can separate the carbon dioxide from the product without additional energy consumption.
Owner:SOUTHEAST UNIV
Method for preparing manganese sulfate, ferrous sulfate and calcium sulfate from manganese ore tailings
InactiveCN102115204AHigh yieldTake advantage ofSolid waste disposalCalcium/strontium/barium sulfatesSolubilitySulfide
The invention provides a method for preparing manganese sulfate, ferrous sulfate and calcium sulfate from manganese ore tailings. The manganese ore tailings are pulverized to powders of 80 meshes to 100 meshes, the powders react with an excessive amount of dilute hydrochloric acid, and the reaction product is filtered to produce a filtrate; an excessive amount of sodium sulfide is added to the filtrate, and the reaction liquid is filtered to produce a filter cake containing manganese sulfide, ferrous sulfide and calcium sulfide; the filter cake containing manganese sulfide, ferrous sulfide and calcium sulfide reacts with a sulfuric acid solution, the reaction product is filtered to produce a filter cake of calcium sulfate and a filtrate containing manganese sulfate and ferrous sulfate, and the filtrate containing manganese sulfate and ferrous sulfate is distilled under a reduced pressure to produce a saturated solution; the saturated solution is cooled at different temperatures according to the difference in solubility between manganese sulfate and ferrous sulfate at different temperatures, so as to produce manganese sulfate crystals and ferrous sulfate crystals respectively; the crystallization product is filtered and dried to obtain a manganese sulfate product and a ferrous sulfate product respectively; and the filter cake of calcium sulfate is washed, dried and pulverized to obtain a calcium sulfate product.
Owner:谢善情
Method for producing calcium sulfide from calcium sulfate
ActiveCN101239706AReaction conditions are easy to controlImprove heat utilizationChemical industryMagnesium/calcium/strontium/barium sulfides/polysulfidesCelsius DegreeCoal
The present invention provides a method for producing sulfurated lime by calcium sulphate and a chemical producing process of sulfurated lime, especially producing sulphuric acid by calcium sulphate and chemical process of sulfurated lime in the production process of clinker. The invention has following steps: a. drying wet calcium sulphate in a drying crusher and material drying preheating system formed by a whirlwind preheater, and ascending the temperature of the material to 150-950 Celsius; b. adding grinded raw coal or coke which thickness is a 0.08mm square opening sieve (screen residue is below 30%) into raw material as a reducer; c. reducing calcium sulphate to sulfurated lime in the reactor containing calcium sulphate and C in the raw material under the impact of the reducing medium; d. mixing sulfurated lime generated by step C and unreacted calcium sulphate; e. So2 obtained by direct transferring reduzate to another reaction, controlling the mol rate of calcium sulphate and sulfurated lime between 6:1 and 1:6. The producing sulfurated lime method has a stable producing course, and a mass production.
Owner:YUNNAN YUNTIANHUA
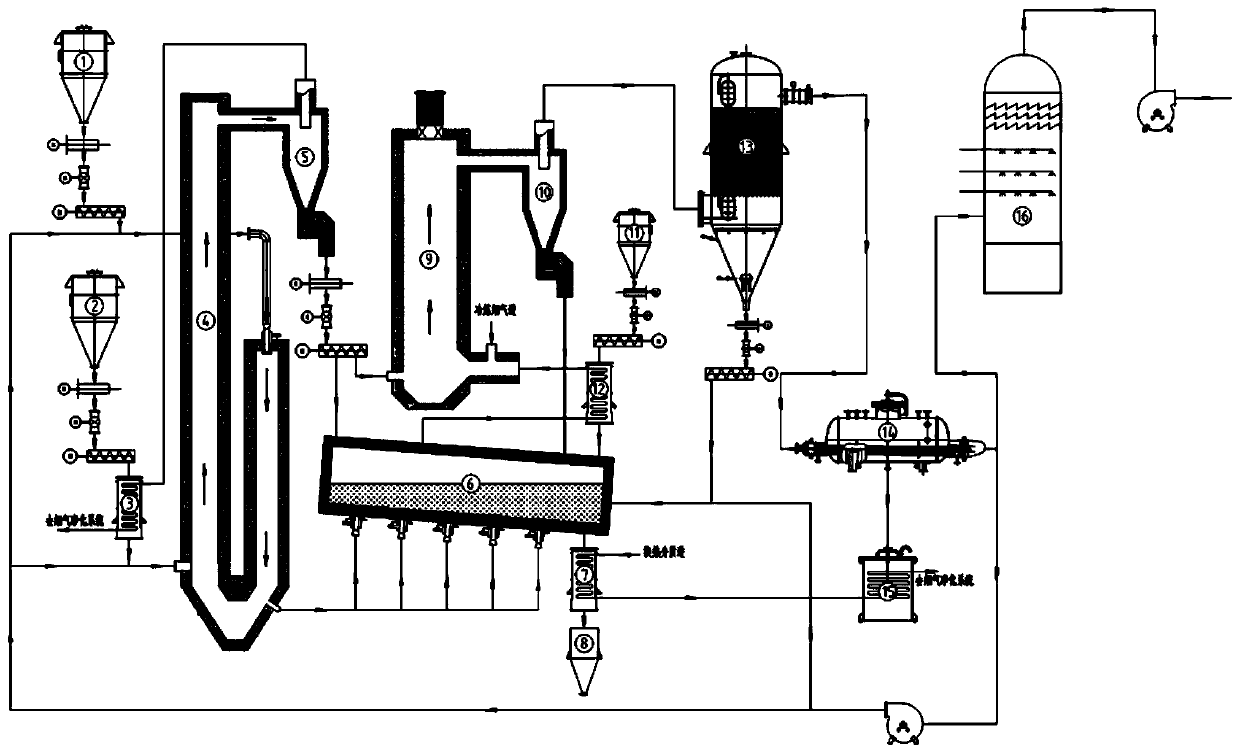
Process for decomposing gypsum through spraying and fluidizing
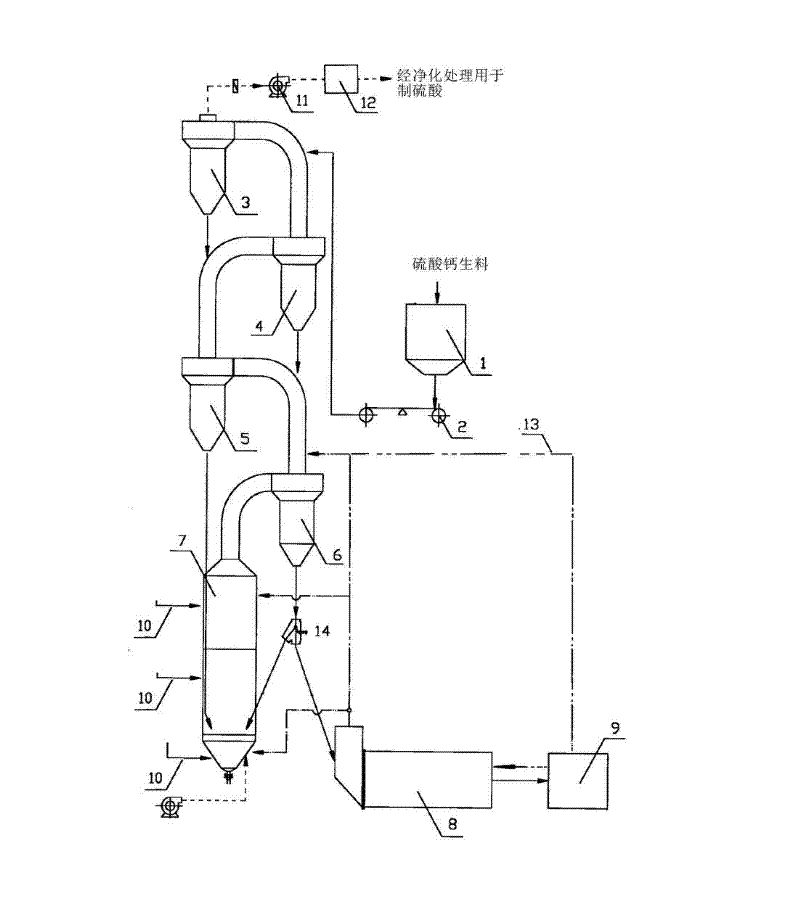
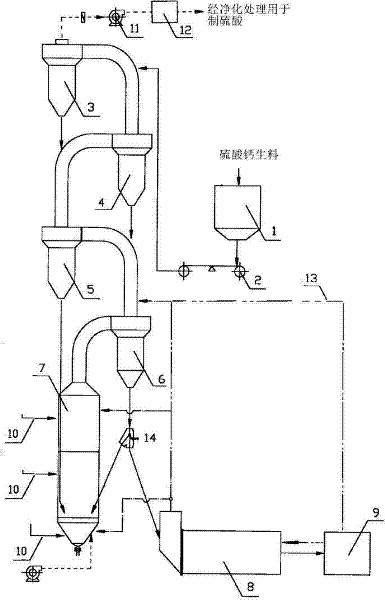
ActiveCN103818884AHigh partial pressure ratioIncrease contact areaChemical industrySulfur compoundsDecompositionCalcium sulfide
The invention relates to the technical field of fluidized decomposition and calcination of gypsum, and particularly discloses a process for producing sulfuric acid by spraying, fluidizing, decomposing and calcining gypsum efficiently. The process comprises the following steps: gasifying brimstone; preheating raw gypsum; spraying, fluidizing and decomposing gypsum to prepare calcium sulfide and sulfur dioxide; preparing sulfur dioxide by calcining and decomposing the prepared calcium sulfide and heated gypsum; preparing sulfuric acid by treating the tail gas. Through the adoption of spraying and fluidizing technology, out-kiln decomposition of gypsum during acid making is realized; the calcium sulfide can be prepared efficiently with low cost; then the calcium sulfide and the preheated gypsum are calcined together for decomposition to prepare the sulfur dioxide. Therefore, the process is absolutely an excellent process for industrialized continuous production, is high in economic benefits and energy utilization ratio.
Owner:SICHUAN HONGDA
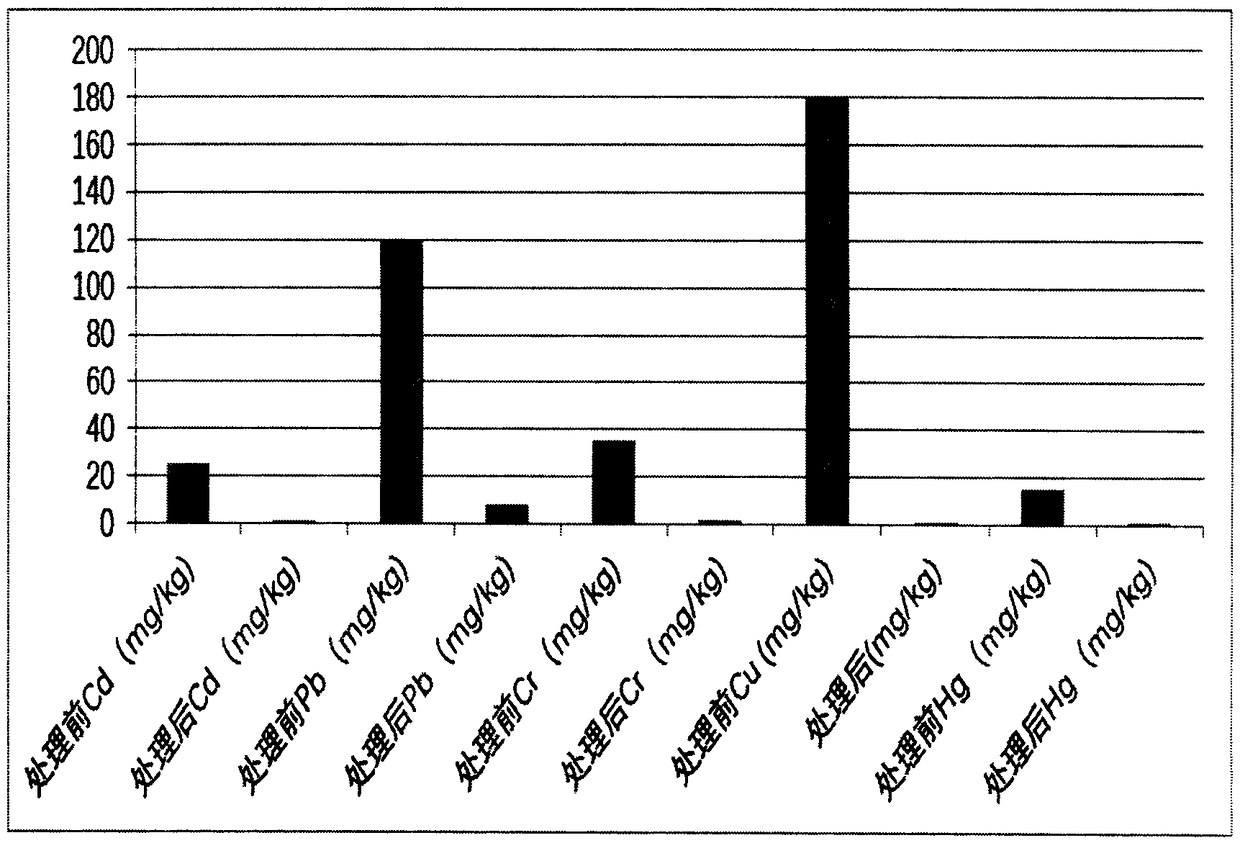
Method for producing heavy metal passivators for farmland soil and application of heavy metal passivators
InactiveCN108410473AEliminate destructionEliminate hazardsAgriculture tools and machinesContaminated soil reclamationSodium BentonitePhosphate
The invention discloses a method for producing heavy metal passivators for farmland soil and application of the heavy metal passivators, and belongs to the field of environmental protection. The method includes steps of adding calcium polysulfide, potassium sulfide, potassium dihydrogen phosphate, sodium lignosulfonate, potassium humate, bentonite and quicklime into a suspending machine, stirringthe calcium polysulfide, the potassium sulfide, the potassium dihydrogen phosphate, the sodium lignosulfonate, the potassium humate, the bentonite and the quicklime and uniformly mixing the calcium polysulfide, the potassium sulfide, the potassium dihydrogen phosphate, the sodium lignosulfonate, the potassium humate, the bentonite and the quicklime with one another to obtain first mixtures; transferring the first mixtures into a ball mill and smashing the first mixtures; adding sodium silicate and EDTA (ethylene diamine tetraacetic acid) into the first mixtures and uniformly stirring the sodium silicate, the EDTA and the first mixtures. The method and the application have the advantages that the heavy metal passivators which are mixtures can be added into the soil contaminated by differenttypes of heavy metal, accordingly, the different types of heavy metal in the soil can be immobilized and passivated and can be turned into insoluble metal salt or alkali, and the activity, the mobility and the bioavailability of the heavy metal in the soil can be deteriorated to a great extent; contamination of the soil due to the different types of heavy metal can be abated, accordingly, the soil productivity and underground water can be protected, and the food safety can be guaranteed.
Owner:吴洪生
Method for removing sodium sulfate in aluminum oxide production process
ActiveCN101182026AAluminates/aluminium-oxide/aluminium-hydroxide purificationAlkali metal carbonatesCalcium sulfideSodium aluminate
The invention discloses a method for removing sodium sulfate in the production process of alumina, relating to a technology for removing sodium sulfate from crystals containing sodium sulfate after evaporation of sodium aluminate solution. It is characterized in that the process of removing sodium sulfate is to reduce the crystalline Na2SO4 in the alumina production process with carbon in the presence of calcium carbonate, so that sulfur can be removed as calcium sulfide, and sodium can be recycled into sodium carbonate. The method of the invention can make the removal rate of sulfur in the sodium sulfate reach more than 60%, and the sodium can be recovered in the form of sodium carbonate.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
Aqueous cutting fluid and preparation method thereof
ActiveCN103666729AGood viscosity and temperatureImprove the lubrication effectLubricant compositionPolyethylene glycolAlkylphenol
The invention discloses an aqueous cutting fluid, which is prepared from the following raw materials in parts by weight: 10-12 parts of polyethylene glycol, 2-3 parts of triethanolamine, 6-9 parts of dibutyl phosphate, 19-22 parts of castor oil, 1-2 parts of calcium sulfide alkylphenol, 1-2 parts of sorbic monostearate, 6-8 parts of assistant and 200 parts of water. The aqueous cutting fluid disclosed by the invention has excellent viscosity-temperature performance, lubricity, extreme pressure property and biodegradability, fast cooling speed, and good corrosion inhibition property, is long in service cycle in comparison with the traditional cutting fluid, small in abrasion on a tool, and high in yield, and has good protection roles on nonferrous metals and black metal, and the processed work-piece is bright.
Owner:广州南星润滑科技股份有限公司
Low-temperature phosphogypsum decomposition method
InactiveCN103130259ALow costWide variety of sourcesCalcium/strontium/barium compoundsSulfur compoundsDecompositionPhosphogypsum
The invention discloses a low-temperature phosphogypsum decomposition method belonging to the field of chemical preparation of phosphorus. In the phosphogypsum decomposition and sulfur / calcium resource recycling process, hydrogen sulfide is used as reducing gas, and calcium chloride is added to decrease the phosphogypsum decomposition temperature by 300-350 DEG C to 650-700 DEG C, thereby effectively reducing the energy consumption in phosphogypsum decomposition, and greatly lowering the phosphogypsum decomposition cost, wherein the gas product sulfur dioxide can be used as raw gas for industrial sulfuric acid preparation, and the solid-phase product is mainly calcium sulfide which can be further purified to serve as an industrial raw material. The decomposition method disclosed by the invention is low in cost and has wide market prospects.
Owner:KUNMING UNIV OF SCI & TECH
Motorcycle clutch iron base friction sheet, preparation process and pairing sheet thereof
The invention provides an iron base friction plate for a motorcycle clutch which is sintered by an iron-base powder metallurgical material. The formula comprises the following compositions of the iron base friction plate in percentage by weight: 1.0 to 6.0 percent of manganese, 0.2 to 1.5 percent of carbon, 1.0 to 5.5 percent of copper, respectively more than 0 but less than 10.0 percent of one or a plurality of compositions of silicon, molybdenum, chromium, nickel, lead, ferric sulfide, tungsten, silicon dioxide, calcium sulfide and asbestos, and the balance being iron, with a density range of the friction plate of between 6.2 and 7.2g / cm<3>. The friction plate uses iron as a base with low cost, which is only one sixth to one tenth of the cost of a copper base material; with the adoption of a powder metallurgy technology, the manufacturing process of the iron base friction plate is simpler than that of the copper base friction plate. A dual disc matched with the friction plate is made of a mild steel disc, and is subject to nitridizing heat treatment or soft nitriding heat treatment on the surface, with a thickness of a nitration case of the dual disc more than or equal to 0.005mm and hardness of the dual disc more than or equal to 350HV0.1. The friction plate can be used under a wet condition, not only has a higher friction coefficient (0.09 to 0.13), but also has excellent wearing resistance, and can be used in the motorcycle clutch.
Owner:重庆市璧山区三泰粉末冶金有限公司 +1
Recycling method of arsenic-containing gypsum slag
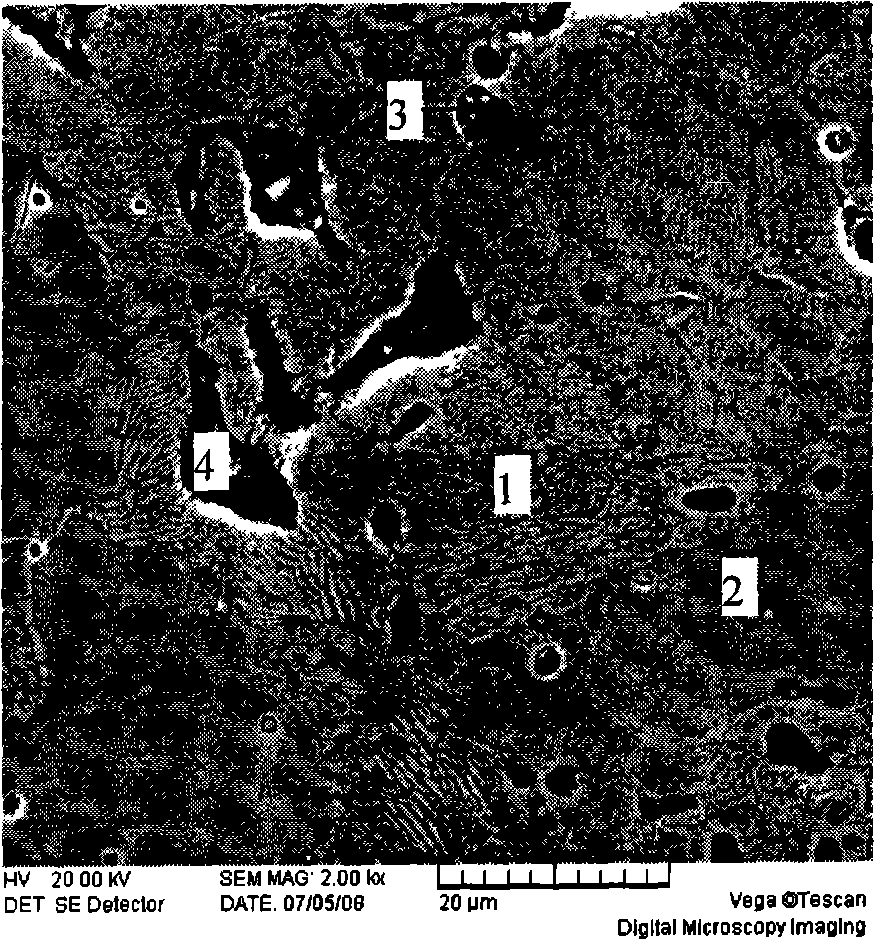
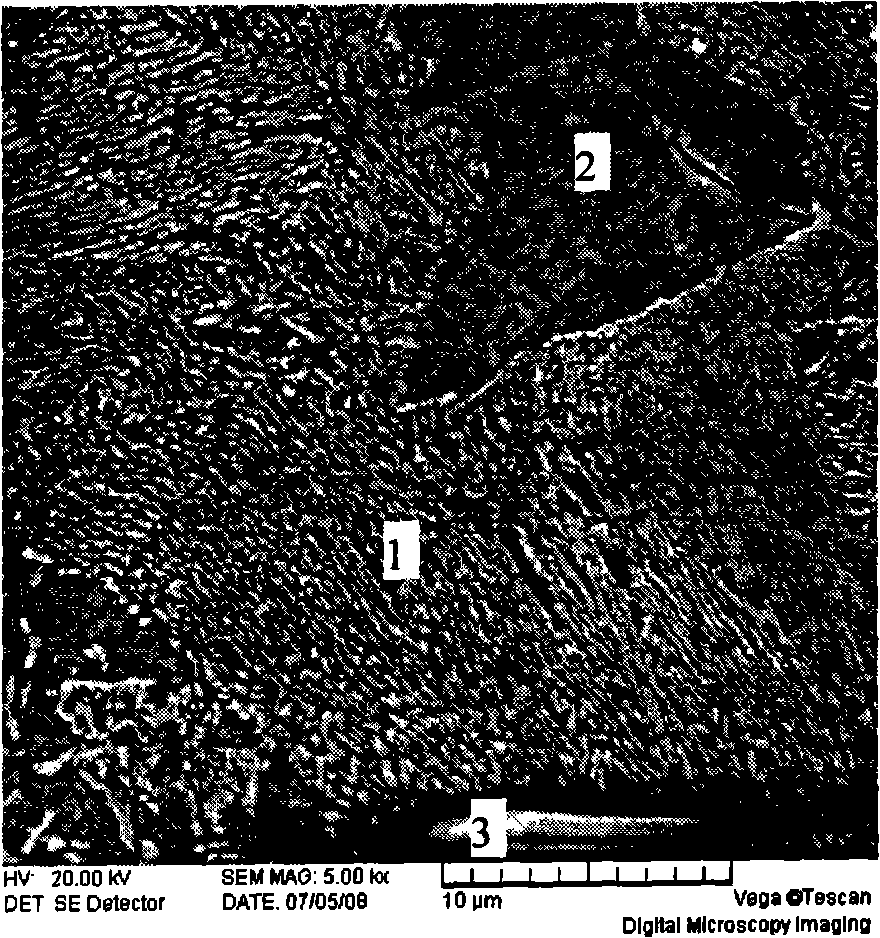
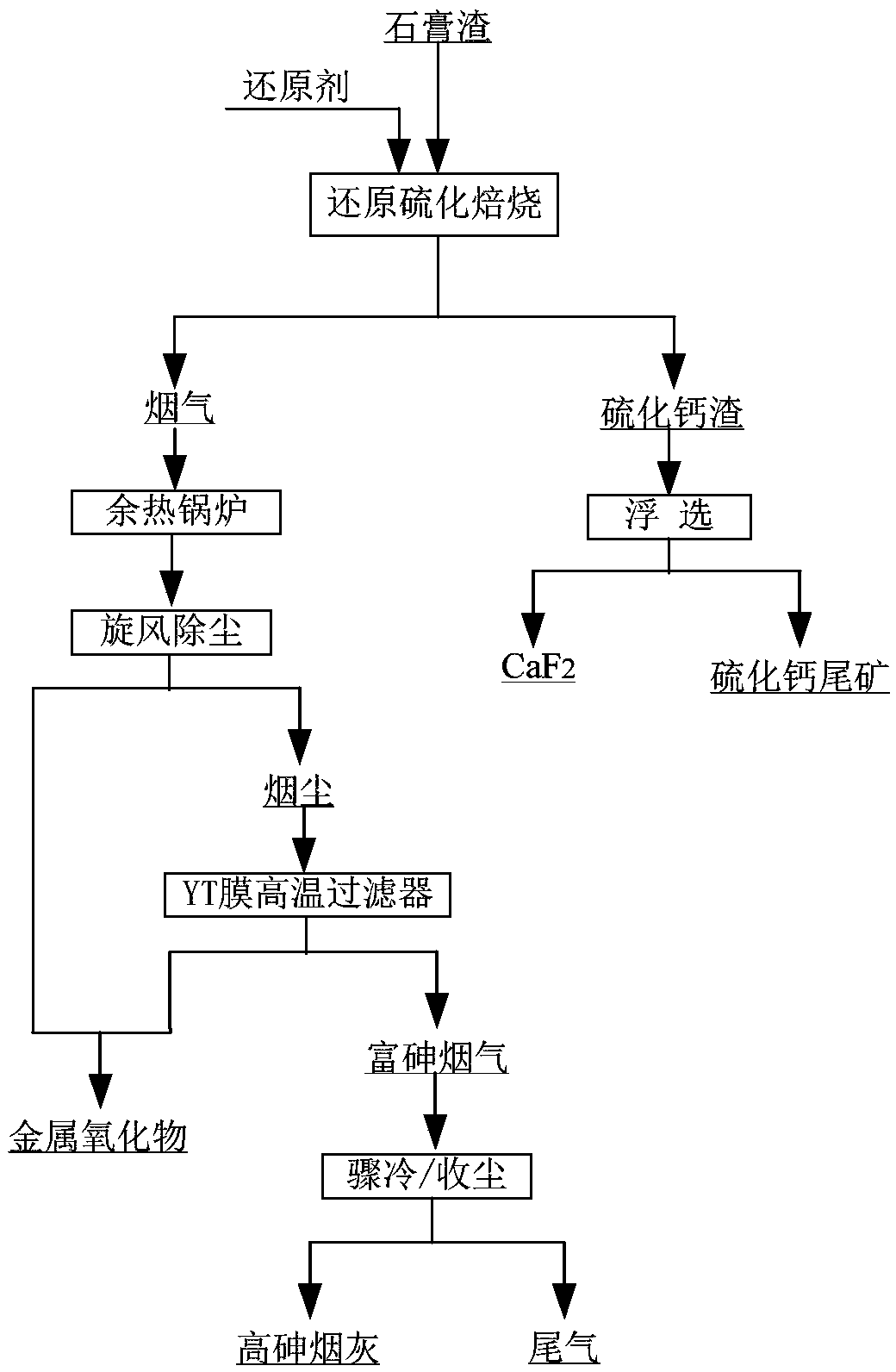
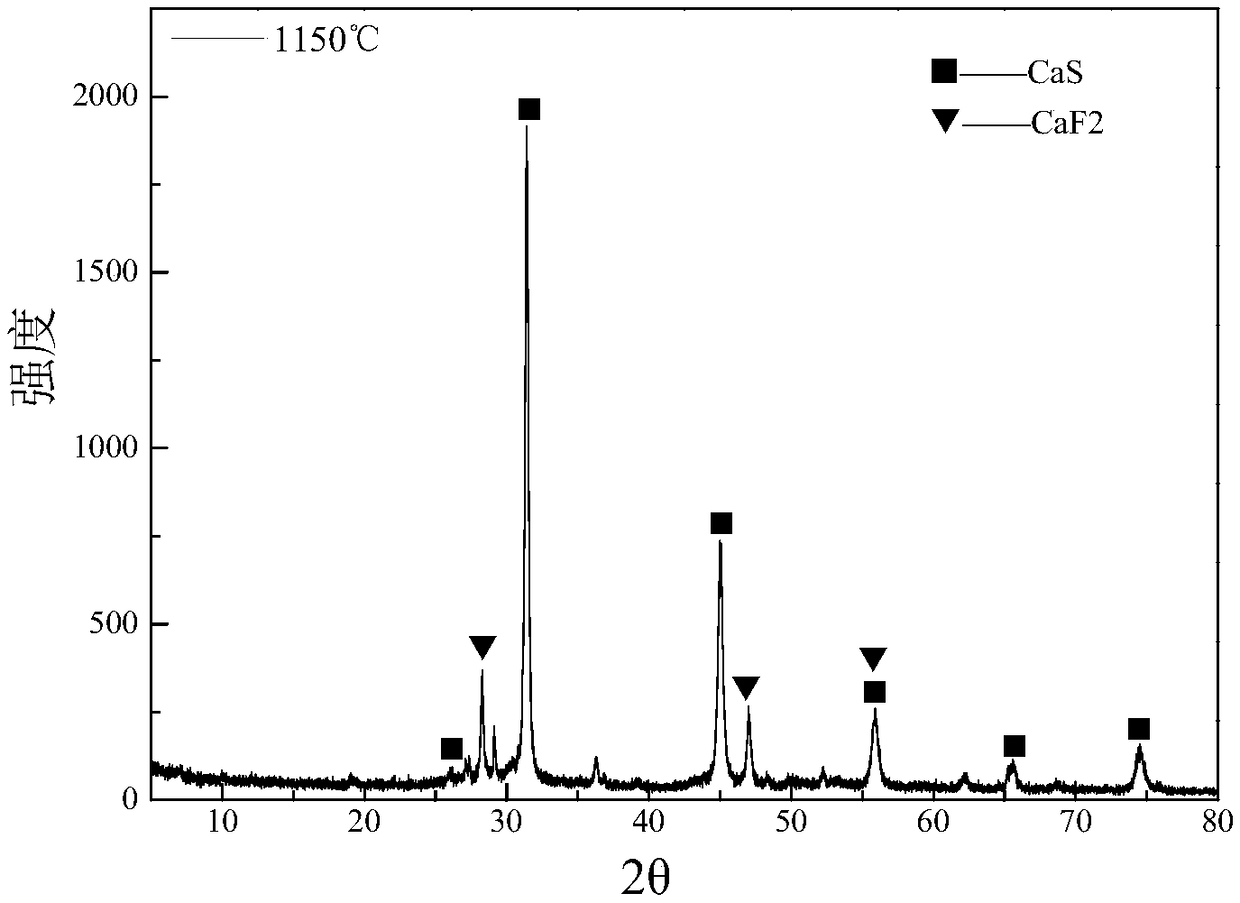
ActiveCN109052331ARealize recyclingAvoid influenceCalcium/strontium/barium fluoridesMagnesium/calcium/strontium/barium sulfides/polysulfidesSlagCalcium sulfide
The invention discloses a recycling method of arsenic-containing gypsum slag. The arsenic-containing gypsum slag is arsenic-containing gypsum slag produced in a waste acid neutralization process. Therecycling method comprises the following steps: (1) carrying out reduction roasting on the arsenic-containing gypsum slag by adopting a carbon reduction agent, so as to obtain arsenic-containing fluegas and roasting slag; (2) carrying out flotation separation on the roasting slag obtained in step (1), so as to obtain calcium sulfide concentrate and calcium fluoride slag. According to the recycling method disclosed by the invention, path opening of arsenic and fluorine in the gypsum slag and recycling of valuable metal are realized; influences caused by the fact that a lot of the arsenic-containing gypsum slag dangerous wastes are stacked and stored on the environment are solved, and the recycling method also has good economic and environmental benefits.
Owner:湖南锐异资环科技有限公司
Calcium lanthanoid sulfide powders, methods of making, and ceramic bodies formed therefrom
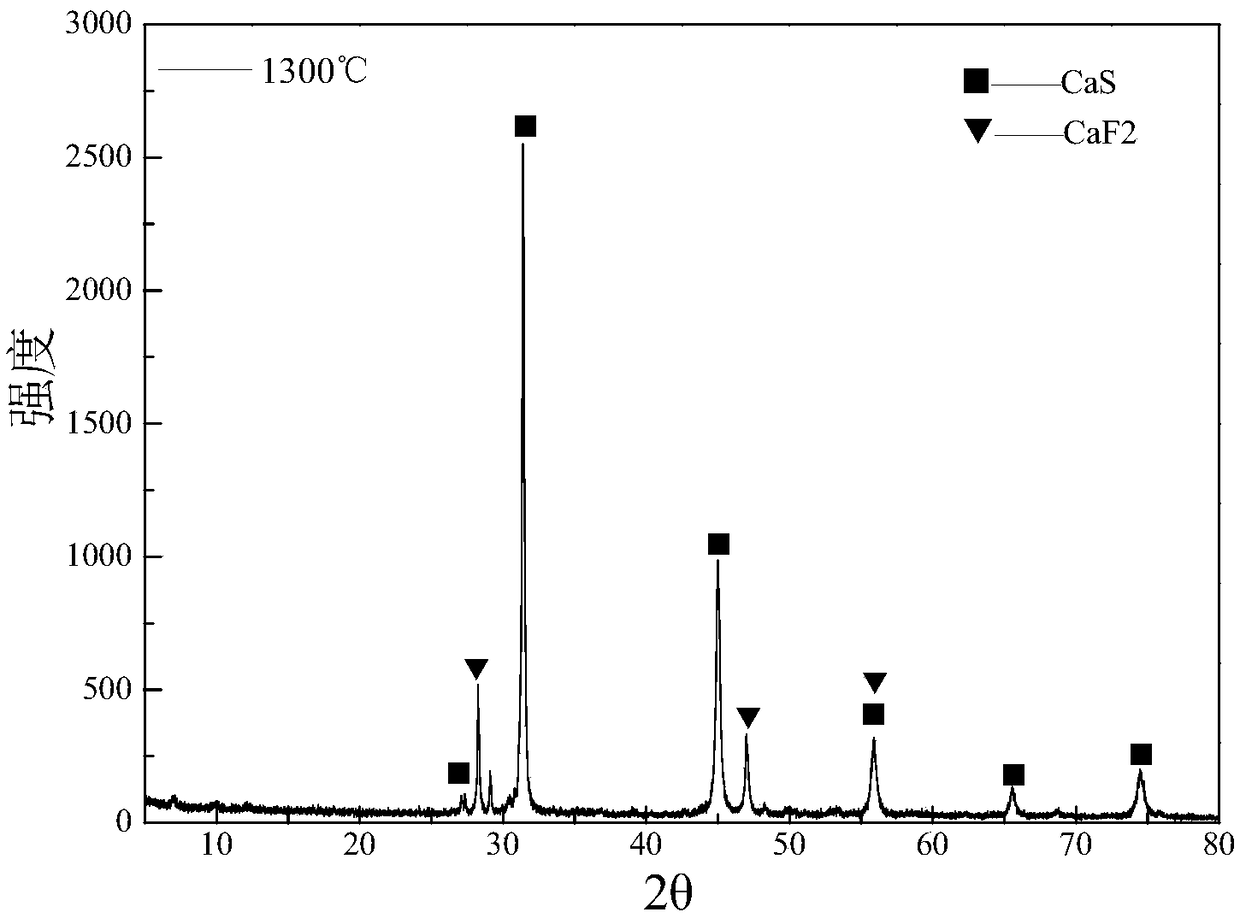
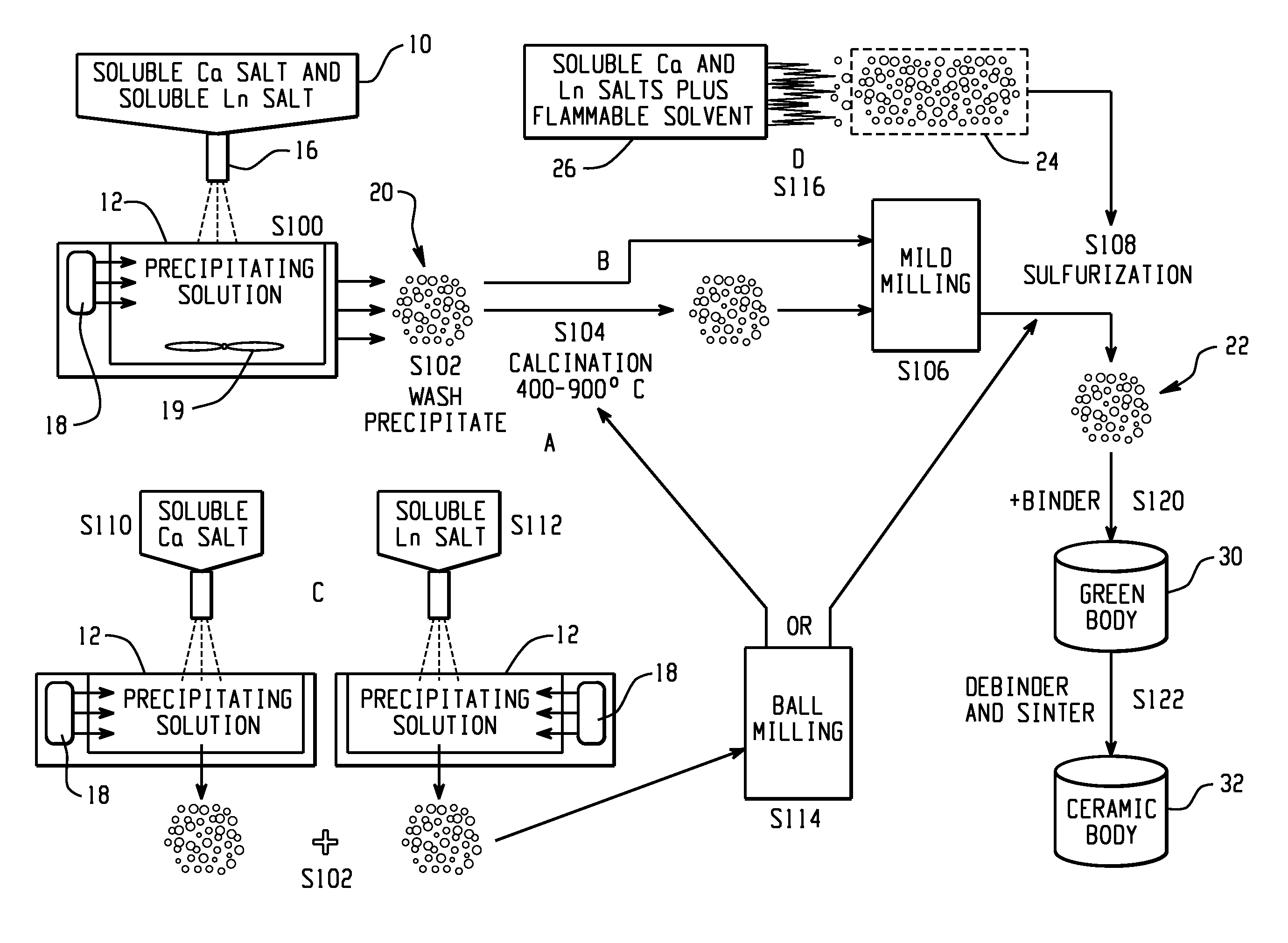
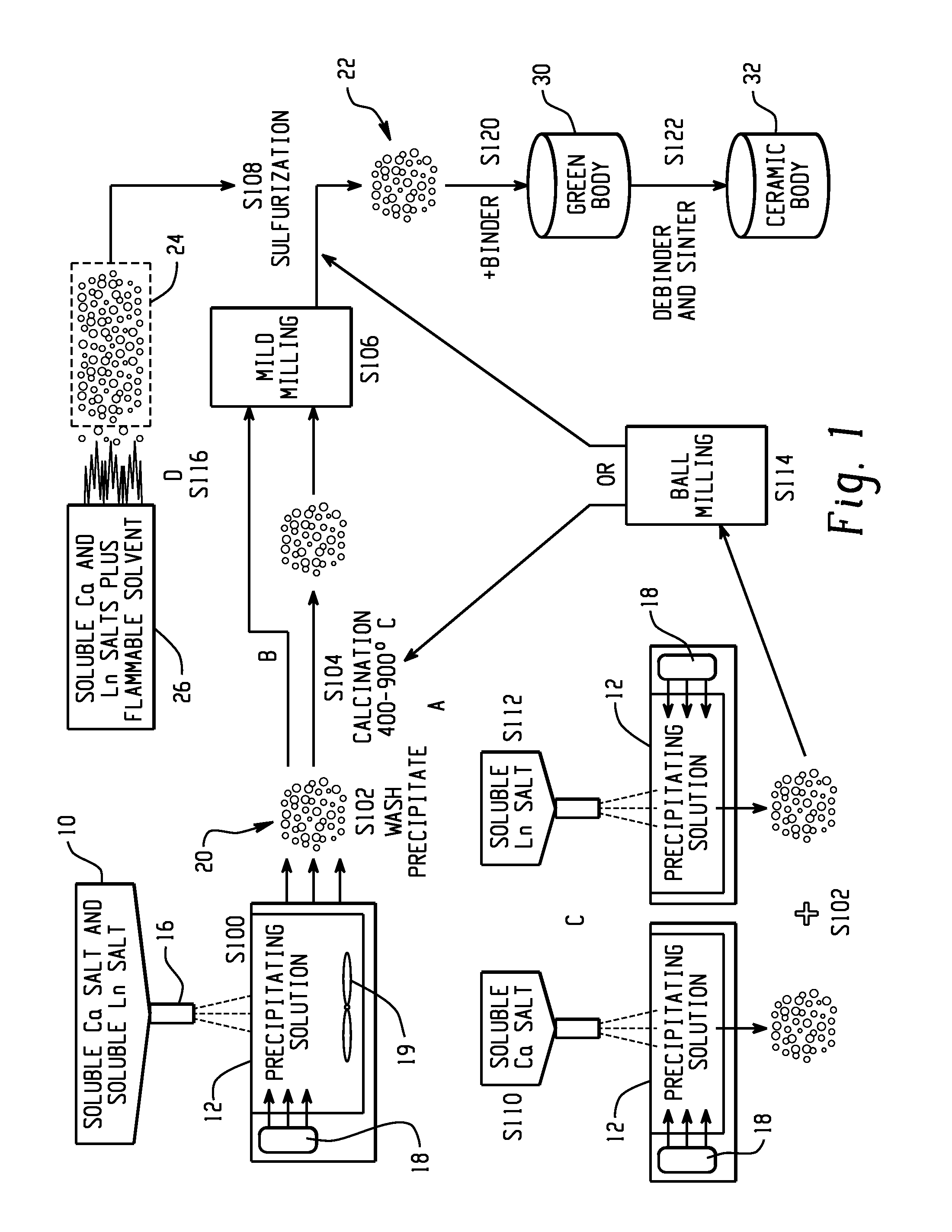
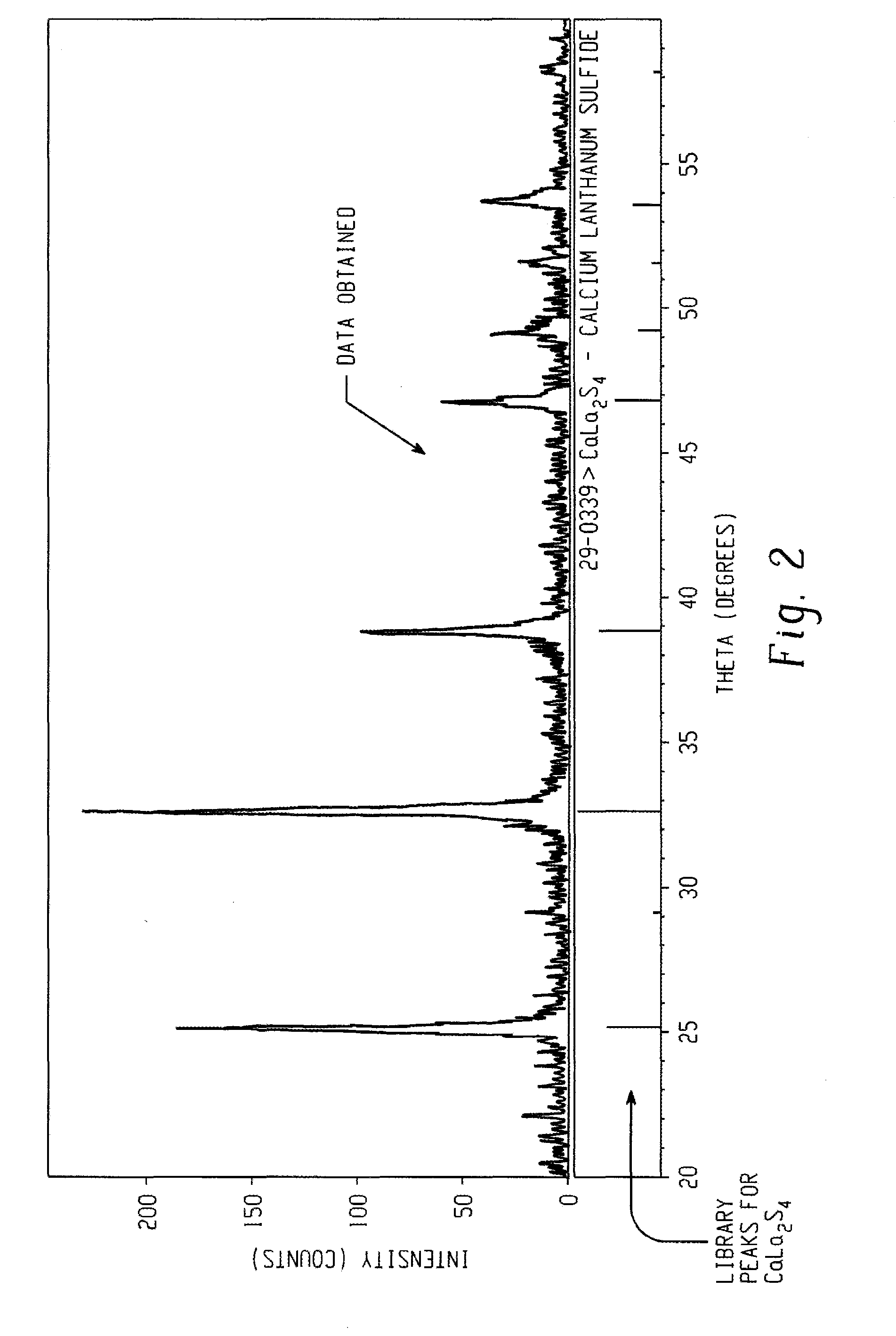
A method of preparing a fine powder of calcium lanthanoid sulfide is disclosed. The method includes spraying soluble calcium and lanthanoid salts into at least one precipitating solution to form a precipitate comprising insoluble calcium and lanthanoid salts, optionally, oxidizing the precipitate comprising insoluble calcium and lanthanoid salts, and sulfurizing the optionally oxidized precipitate to form a fine powder of calcium lanthanoid sulfide. An alternative method for forming the powder is by flame pyrolysis. The calcium lanthanoid sulfide powder produced by either method can have an impurity concentration of less than 100 ppm, a carbon concentration of less than 200 ppm, a BET surface area of at least 50 m2 / g, and an average particle size of less than 100 nm.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Method for preparing caustic soda and coproducing calcium carbonate by using waste alkali liquid generated during ethylene cracking
InactiveCN102180486AEfficient governanceEfficient use ofCalcium/strontium/barium carbonatesMultistage water/sewage treatmentChemical reactionPetrochemical
The invention discloses a method for preparing caustic soda and coproducing calcium carbonate by using waste alkali liquid generated during ethylene cracking and relates to the technical field of comprehensive treatment and utilization of three wastes produced during ethylene cracking in petrochemical industry. The method comprises the steps of: heating waste alkali liquid generated during ethylene cracking, taking waste alkali liquid after all oil and soap are melted and float on the waste alkali solution; reacting the sodium sulfide in the obtained waste alkali liquid with calcium hydroxide in a glass lining reactor, starting a stirrer so that the solution has chemical reaction to produce mixture solution mainly containing sodium hydroxide; filtering to obtain a white calcium carbonate filter cake, washing and drying, crushing, separating and packaging to respectively obtain calcium carbonate and calcium sulfide products; sending the obtained clear sodium hydroxide alkali liquid in a normal-pressure evaporator, evaporating excessive moisture till the solution is in a saturated state, and then discharging, cooling into a block, and packaging to obtain a blocky caustic soda product. The method not only can lower the treatment cost of organic chemical industry and recycle waste material to create economic benefits, and but also can directly eliminate pollution sources.
Owner:何侠
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com