Method for absorbing sulfur dioxide and co-producing sulphur
A sulfur dioxide and sulfur technology, applied in chemical instruments and methods, separation methods, preparation/purification of sulfur, etc., can solve problems such as low absorption efficiency and comprehensive utilization rate, harsh reaction conditions, complex processes and equipment, etc., to achieve comprehensive Utilization, mild reaction conditions, and high-efficiency absorption effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
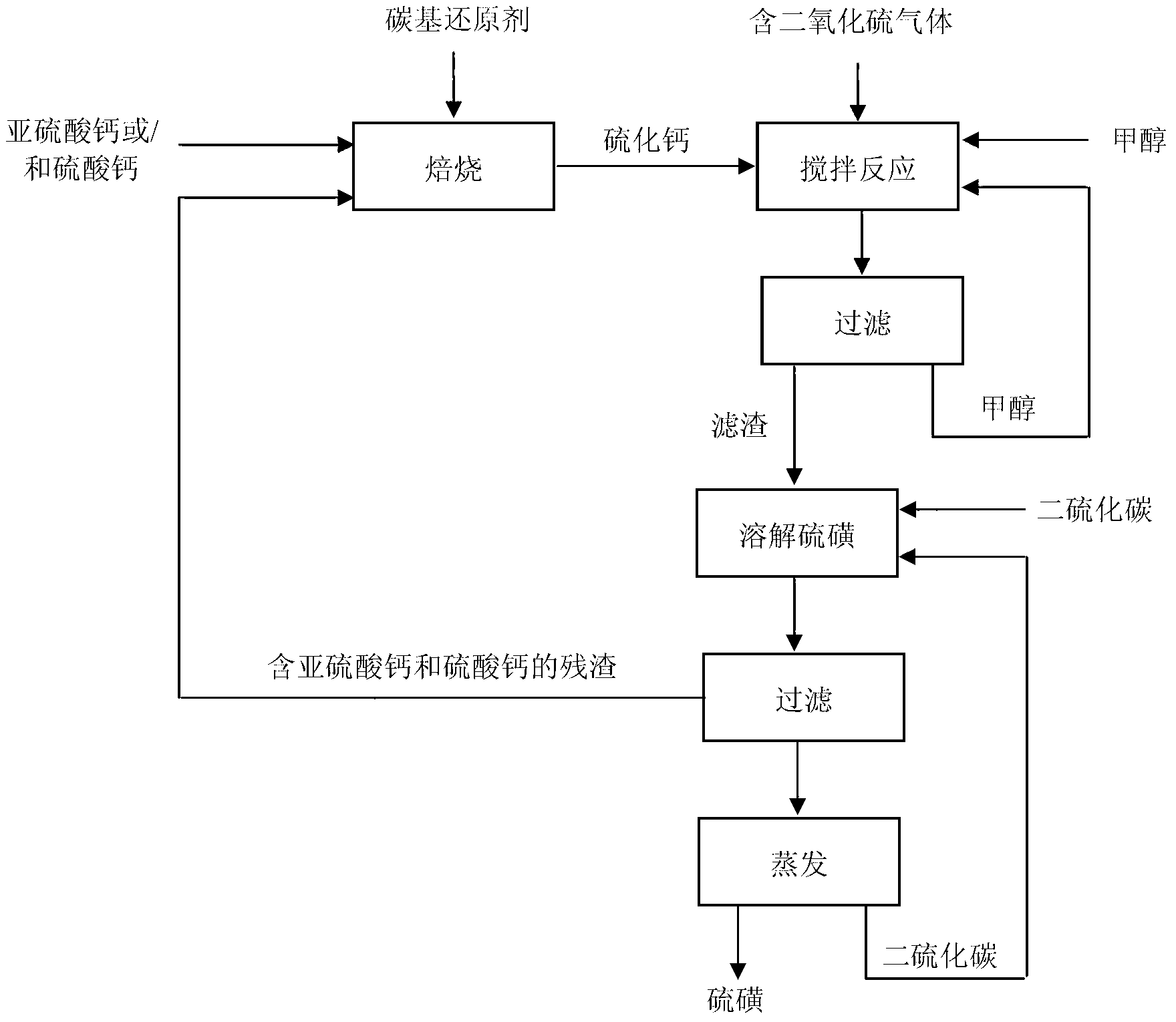
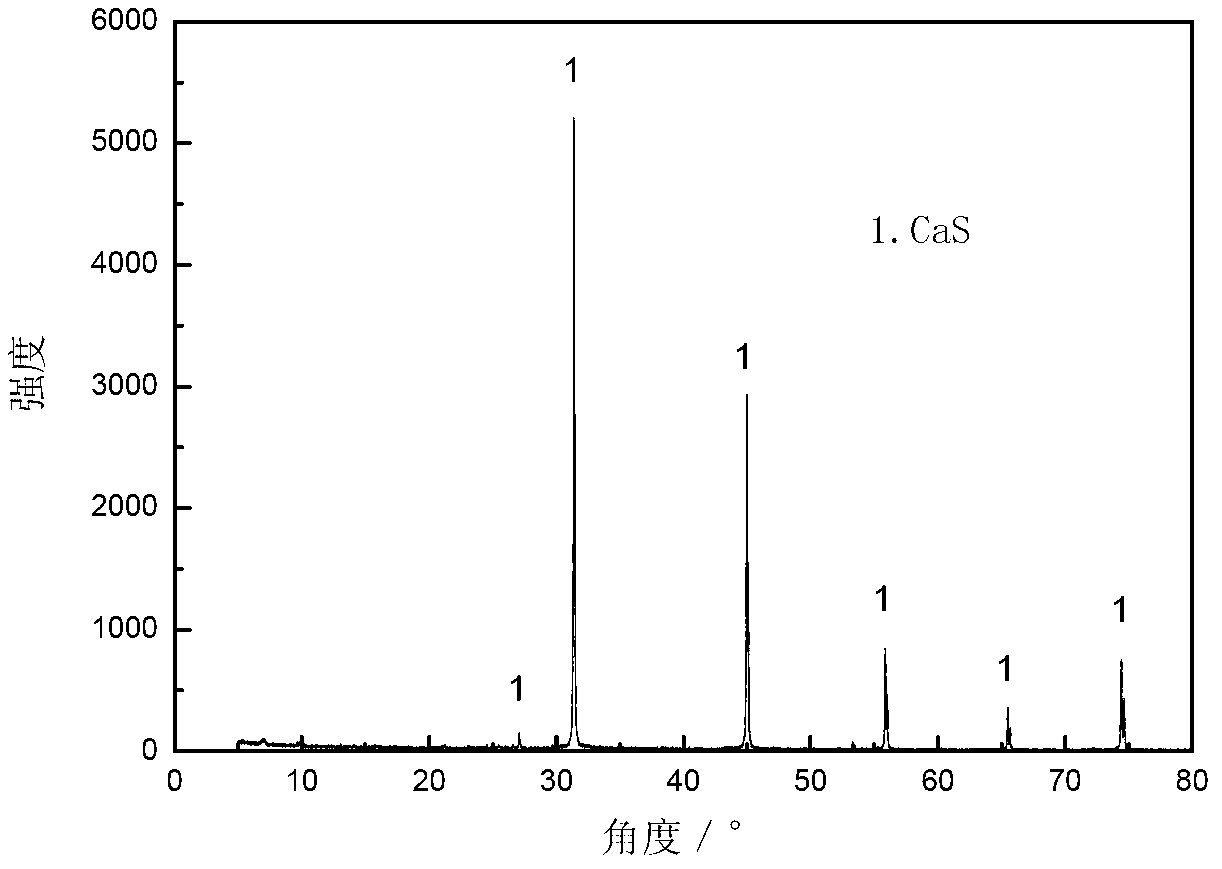
[0036] (1) In a closed reactor, charcoal is used as a reducing agent, the molar ratio of carbon to calcium sulfite in charcoal is 2.5:1, the reaction temperature is 900°C, and the reaction time is 1.5h, to obtain calcium sulfide. The XRD pattern is shown in figure 2 ;
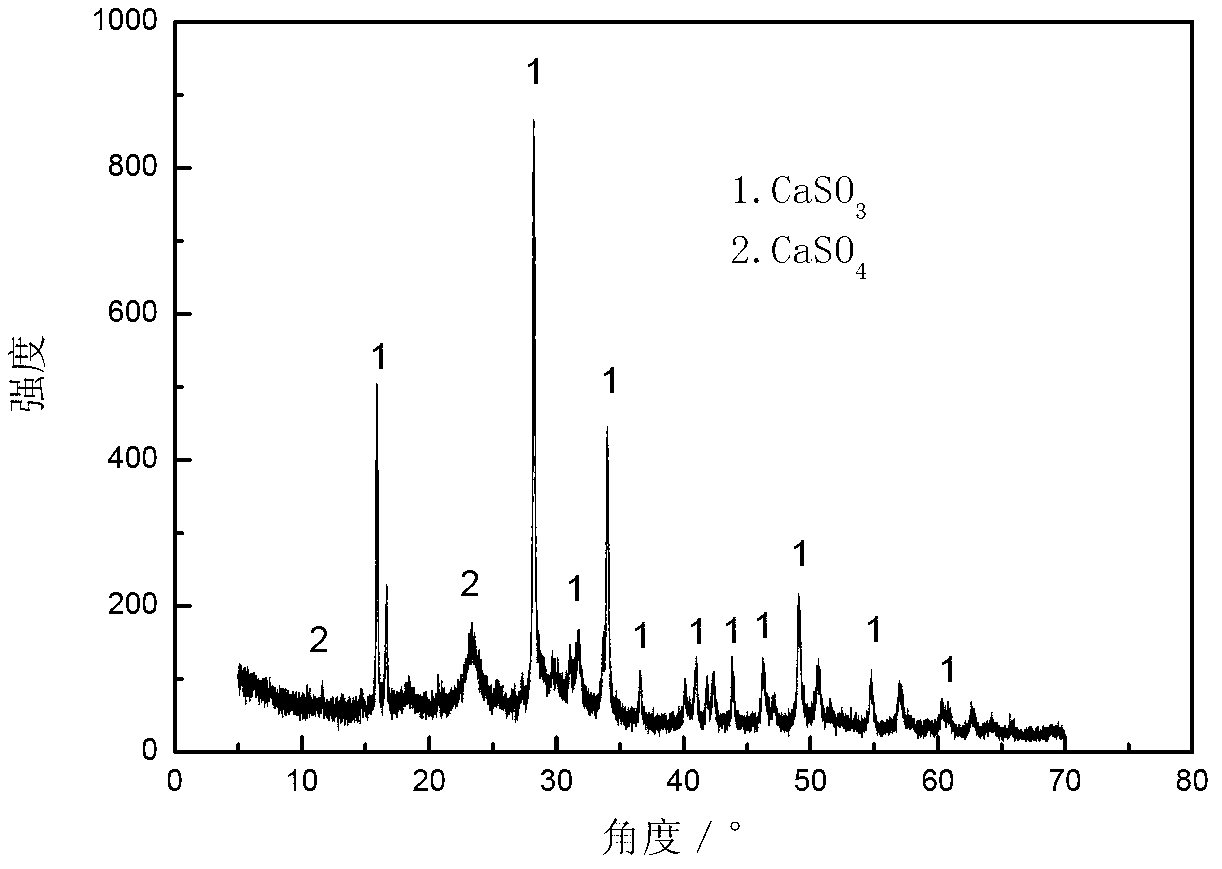
[0037] (2) In a closed reactor, use methanol as a solvent, add calcium sulfide, control the mass ratio of solvent to calcium sulfide to be 5:1, feed sulfur dioxide under stirring conditions, and control the amount of SO 2 The molar ratio to calcium sulfide is 1.5:1, stirred and reacted at 20°C for 12h, filtered to separate the filtrate from the solid reaction product, and the filtrate was recycled as a solvent, the solid reaction product was dissolved by carbon disulfide, and the filtrate and filter residue were obtained by filtration (see XRD pattern in image 3 ), the filter residue is recycled as a raw material for the preparation of calcium sulfide, and the filtrate is evaporated to obtain a solid sulfur p...
Embodiment 2
[0039] (1) In a closed reactor, coal is used as a reducing agent, the molar ratio of carbon in coal to calcium sulfite is 2.5:1, the reaction temperature is 900°C, and the reaction time is 1.5 hours to obtain calcium sulfide;
[0040] (2) In a closed reactor, use ethanol as a solvent, add calcium sulfide, control the mass ratio of solvent to calcium sulfide to be 4:1, feed sulfur dioxide under stirring conditions, and control the amount of SO 2The molar ratio with calcium sulfide is 1.5:1, stirred and reacted at 30°C for 10h, filtered to separate the filtrate from the solid reaction product, and the filtrate was recycled as a solvent, the solid reaction product was dissolved by carbon disulfide, filtered to obtain the filtrate and filter residue, and the filter residue was used as a preparation The raw material of calcium sulfide is recycled, and the filtrate is evaporated to obtain a solid sulfur product. The sulfur dioxide absorption rate is 87.46%, and the sulfur yield rate...
Embodiment 3
[0042] (1) In a closed reactor, coal is used as the reducing agent, the molar ratio of carbon element in coal to calcium sulfate is 3:1, the reaction temperature is 1000°C, and the reaction time is 1.5h to obtain calcium sulfide;
[0043] (2) in such as Figure 5 In the reaction device shown, methanol is used as a solvent in each reactor, calcium sulfide is added, the mass ratio of solvent to calcium sulfide is controlled to be 5:1, and the sintering flue gas of a steel plant in Hunan is passed into the sintering flue gas under stirring conditions. The concentration of sulfur dioxide is 1500mg / m 3 At the beginning, valves 2, 5, 7, and 9 are closed, and valves 1, 3, 4, 6, and 8 are opened, so that the flue gas passes through the primary reactor 10 and the secondary reactor 11 in turn, and waits for the exhaust gas in the primary reactor 10 After the calcium sulfide reaction is complete, switch the valves to: close valves 1, 3, 5, and 8, open valves 2, 4, 6, 7, and 9, so that t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com