Preparation for metal sulfide
A metal sulfide, sulfide technology, applied in the preparation of sulfide/polysulfide, alkali metal sulfide/polysulfide, magnesium/calcium/strontium/barium sulfide/polysulfide, etc. Obtain high-purity products, and it is unlikely to prepare metal sulfides in large quantities, so as to achieve the effects of easy control of reaction conditions, reduced risk, and favorable mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
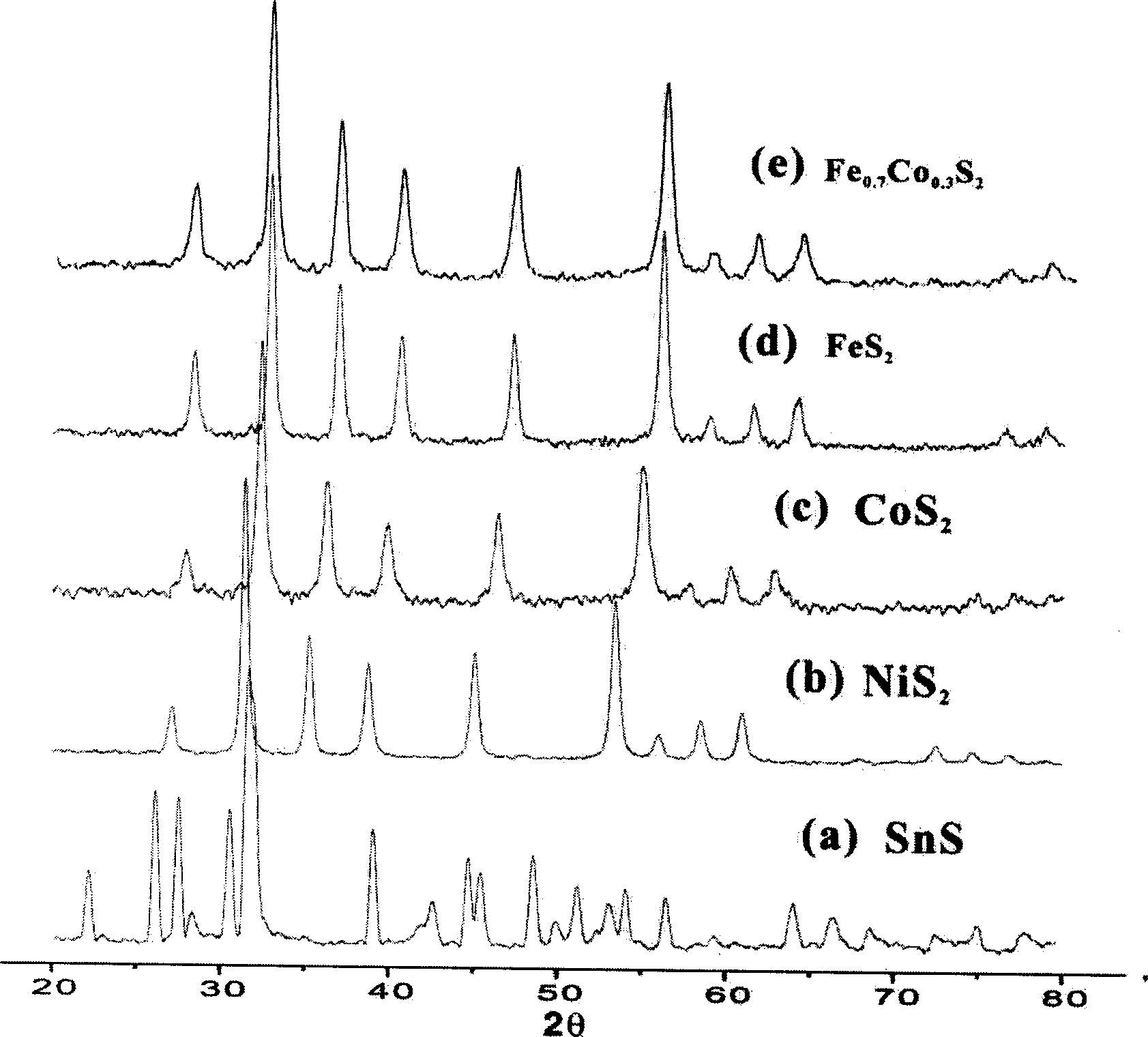
[0030] Example 1: Place 16 grams of elemental copper powder and elemental sulfur in an atomic ratio of 2:1 in a 30ml autoclave, add acetone to 90% of the reactor, and keep the reactor at 110°C for 3 days at 20 atmospheres. After natural cooling, the product powder was taken out by filtration, washed with carbon disulfide, and dried to obtain Cu 2 S 15.3 g, X-ray analysis showed that the product was Cu 2 S.
example 2
[0031] Example 2: Put 12.8 grams of elemental Sn powder and elemental sulfur in an atomic ratio of 1:1.03 in a 30ml autoclave, and add toluene to 90% of the reactor. The reaction kettle was kept at a constant temperature of 220°C for 3 days under about 300 atmospheric pressure. After natural cooling, the product powder was filtered out, washed with carbon disulfide, and dried to obtain 12.2 grams of SnS. The X-ray analysis results showed that the product was SnS.
example 3
[0032] Example 3: 10 grams of metal nickel powder and 11.3 grams of elemental sulfur were placed in a 50 ml autoclave, and toluene was added to reach 90% of the reactor. The reaction kettle was kept at about 300 atmospheres at 220°C for 5 days, and after natural cooling, the product powder was filtered out, washed with carbon disulfide, and dried to obtain NiS 2 20.2 grams, X-ray and EDX analysis showed that the product was NiS 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com