Lithium ion battery electrolyte
A lithium-ion battery and electrolyte technology, applied in secondary batteries, circuits, electrical components, etc., can solve problems such as damage to the structure of graphite sheets, unfavorable performance of graphite, and peeling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of lithium-ion battery electrolyte: mix propylene carbonate (PC), ethylene carbonate (EC) and diethyl carbonate (DEC) in a ratio of 1:1:1 (mass ratio) to make 1mol / L LiPF 6 To the electrolyte solution, add 2% (by mass) of N-methylsulfonylphthalimide (the structural formula is shown below) to obtain the lithium-ion battery electrolyte solution.
[0044]
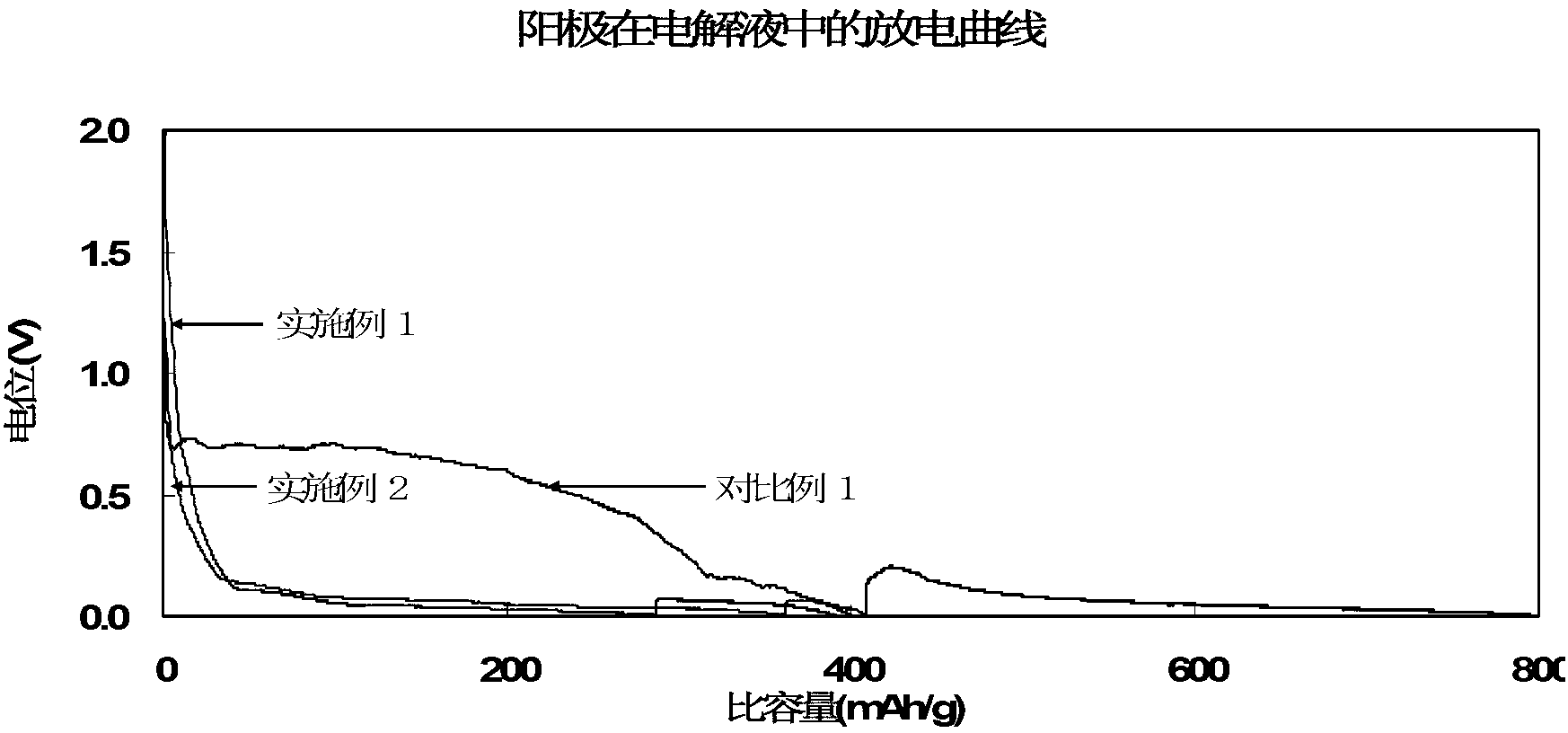
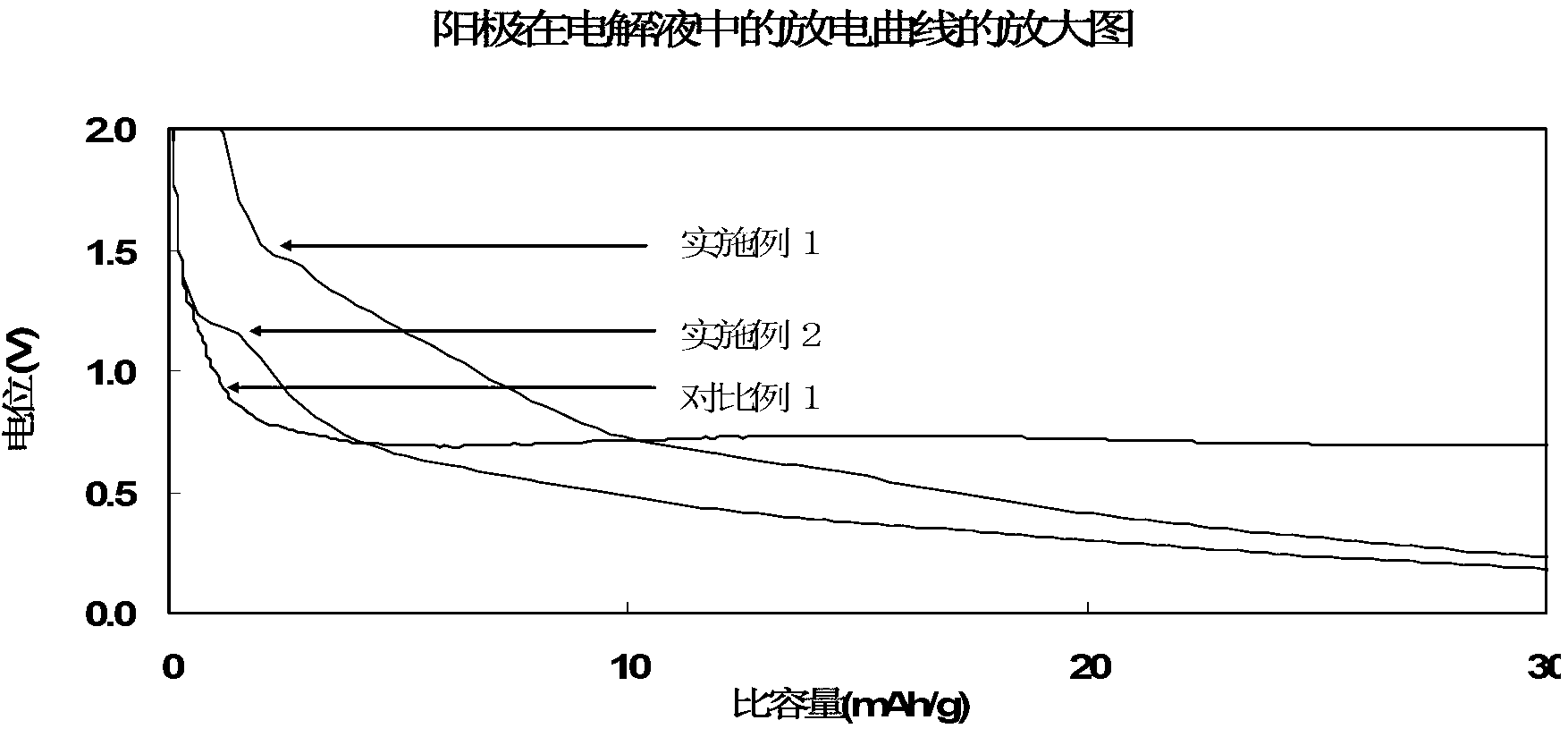
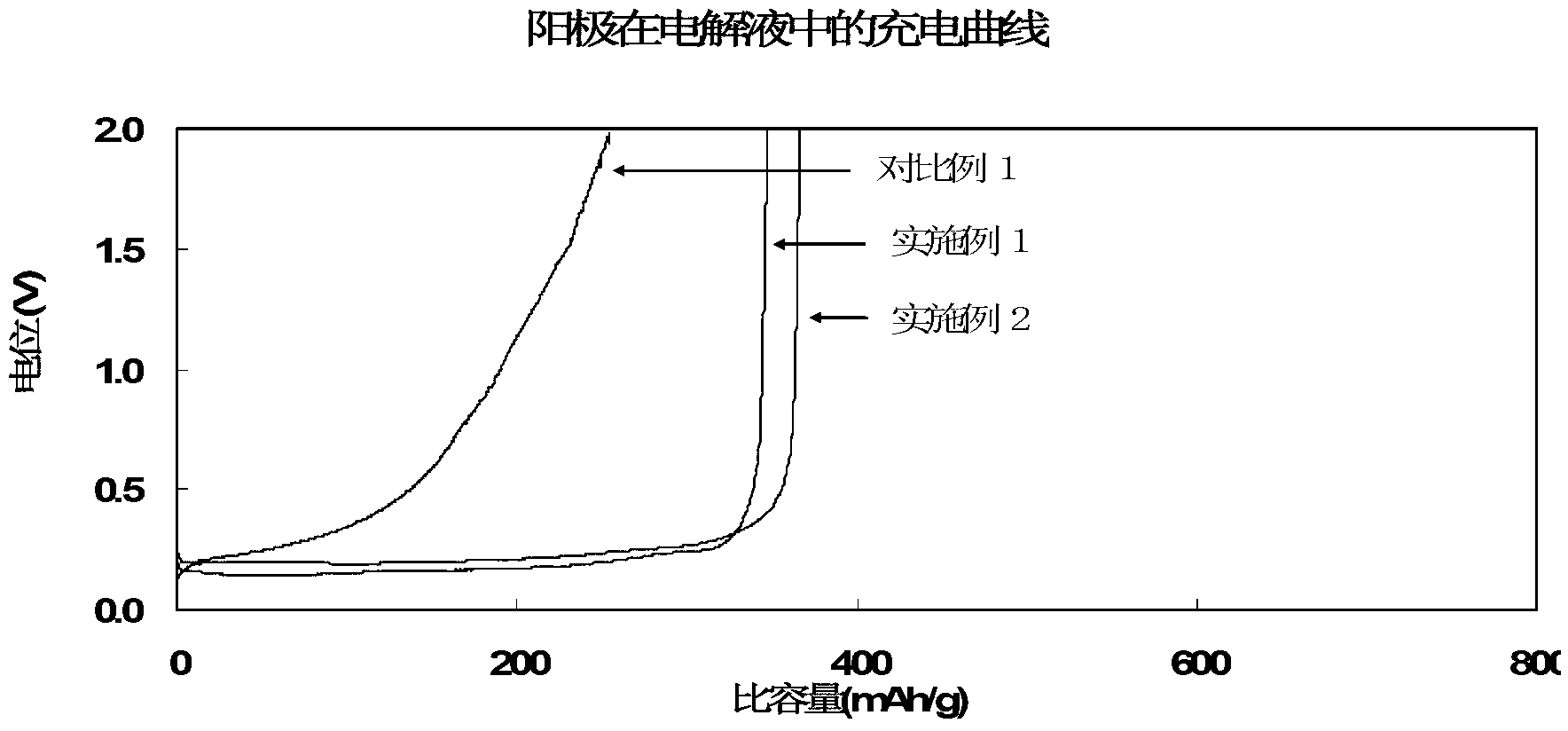
[0045] Preparation of lithium-ion battery: inject the electrolyte solution prepared above into a 2430 button battery. The graphite used is natural graphite, the weight of the pole piece coated on the copper current collector is 13.9 mg, the mass content of the graphite active material in the pole piece is 96.5%, and the counter electrode is a lithium piece. For this button, discharge at 0.05C to 0.005V, and then discharge at 0.05mA to 0.005V. This is the process of lithium ions intercalating into graphite. The capacity of this process is called lithium intercalation capacity of graphite, and the lithium i...
Embodiment 2
[0049] Prepare lithium-ion battery electrolyte: mix propylene carbonate (PC), ethylene carbonate (EC) and diethyl carbonate (DEC) in a ratio of 1:1:1 (mass ratio) to make 1mol / L LiPF6 electrolyte, add 5% (by mass) of N-methylsulfonylsuccinimide (the structural formula is shown below), that is, the lithium-ion battery electrolyte is prepared.
[0050]
[0051] Preparation of lithium-ion battery: inject the electrolyte solution prepared above into a 2430 button battery. The graphite used is natural graphite, the weight of the pole piece coated on the copper current collector is 13.9 mg, the mass content of the graphite active material in the pole piece is 96.5%, and the counter electrode is a lithium piece. For this button, discharge at 0.05C to 0.005V, and then discharge at 0.05mA to 0.005V. This is the process of lithium ion intercalation into graphite. The capacity of this process is called lithium intercalation capacity of graphite, and the lithium intercalation capacity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com