Bi-imidazole ring functional ionic liquid, preparing method thereof, electrolyte and lithium secondary battery
A technology of functional ionic liquid and biimidazole ring, applied in secondary batteries, non-aqueous electrolyte batteries, lithium batteries, etc., can solve the problems of affecting conductivity, electrochemical window compatibility, affecting battery performance, and large interface impedance, etc. Achieve high practical application value, improve safety, and effect of high thermodynamic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
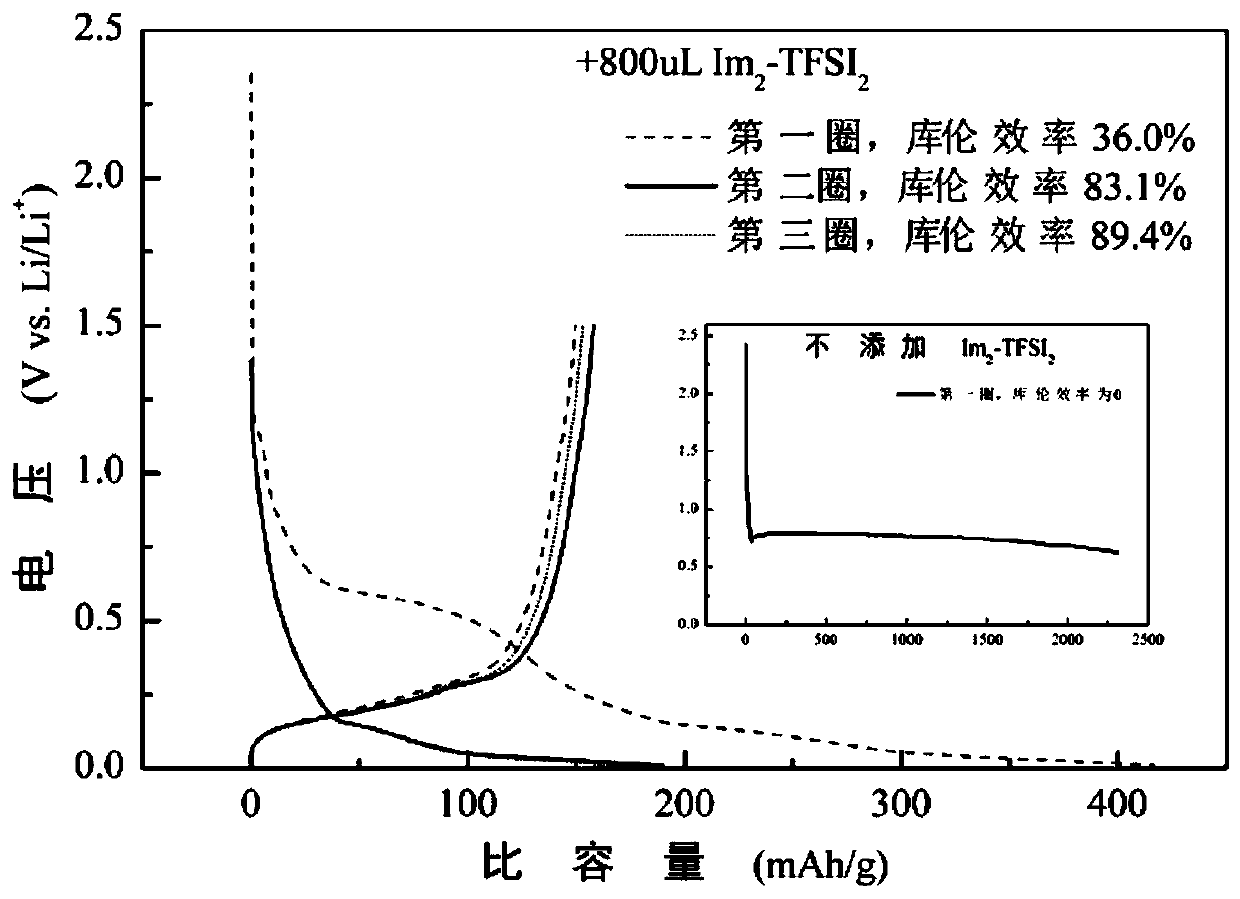
[0045] Embodiment 1: Biimidazole ring functional ionic liquid Im 2 -TFSI 2 and a single ionic liquid electrolyte and graphite half-cell
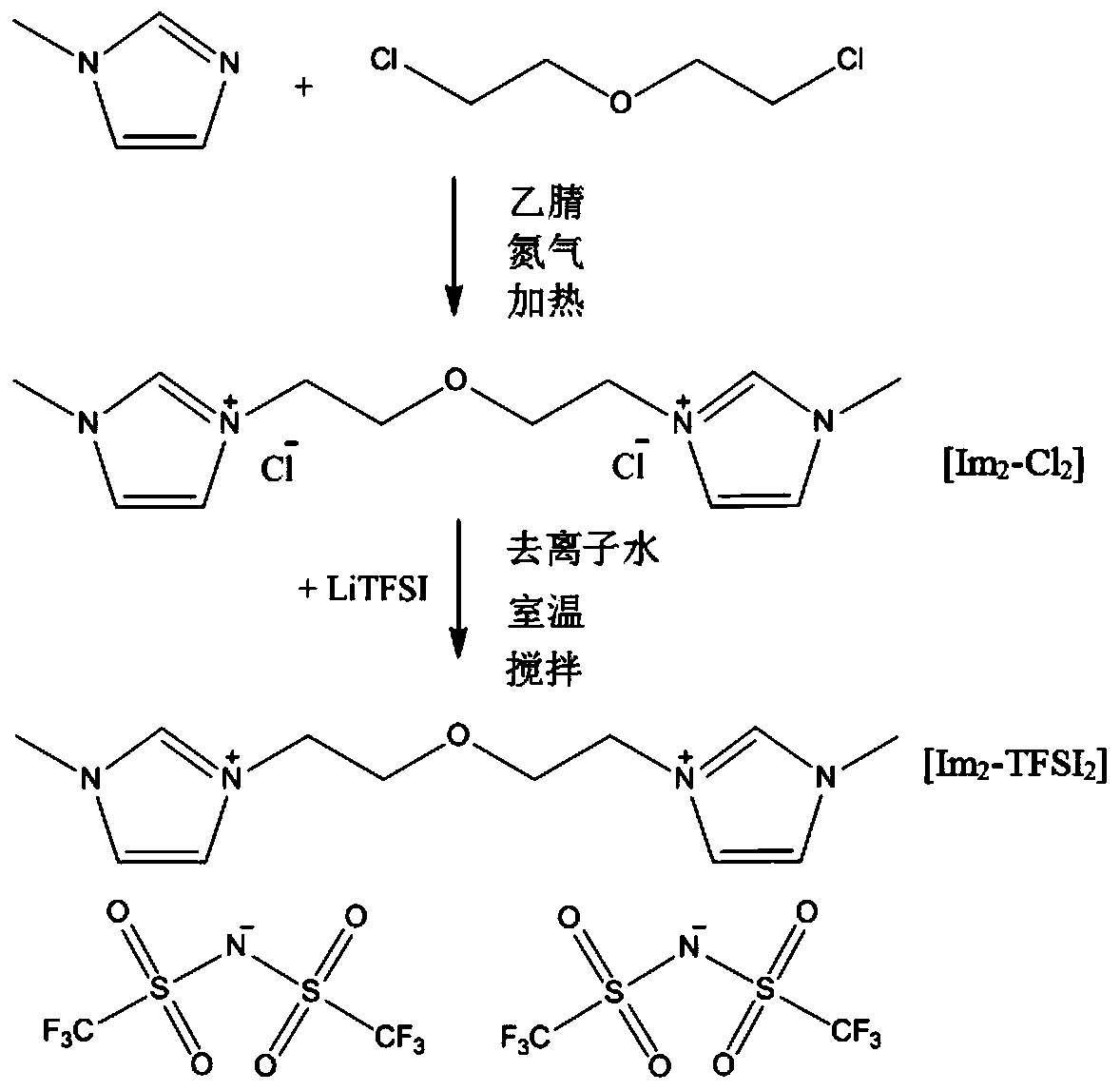
[0046] The double imidazole ring functional ionic liquid Im involved in the present embodiment 2 -TFSI 2 prepared as figure 1 As shown, the specific steps are as follows:
[0047] (1) Add 50mL of acetonitrile and 0.2mol N-methylimidazole into a three-necked flask, put in a magnet, heat and magnetically stir in an oil bath at 80°C, reflux and condense, and nitrogen gas is introduced. After 30min, use a constant pressure funnel to Add 0.12mol 2,2'-dichloroethylether dropwise and react for 24 hours; pour the reaction product into a round-bottomed flask, and evaporate at 60°C for more than 2 hours, then place the product in a vacuum oven at 60°C for more than 24 hours to obtain intermediate product, named Im 2 -Cl 2 ;
[0048] (2) Take 0.05mol Im 2 -Cl 2 Dissolve in 25mL deionized water, stir well; another 0.12mol LiTFSI is dissolved i...
Embodiment 2
[0055] Embodiment 2: Biimidazole ring functional ionic liquid Im 2 -TFSI 2 and multicomponent ionic liquid electrolytes and graphite half-cells
[0056] The double imidazole ring functional ionic liquid Im involved in the present embodiment 2 -TFSI 2 The preparation method is with embodiment 1.
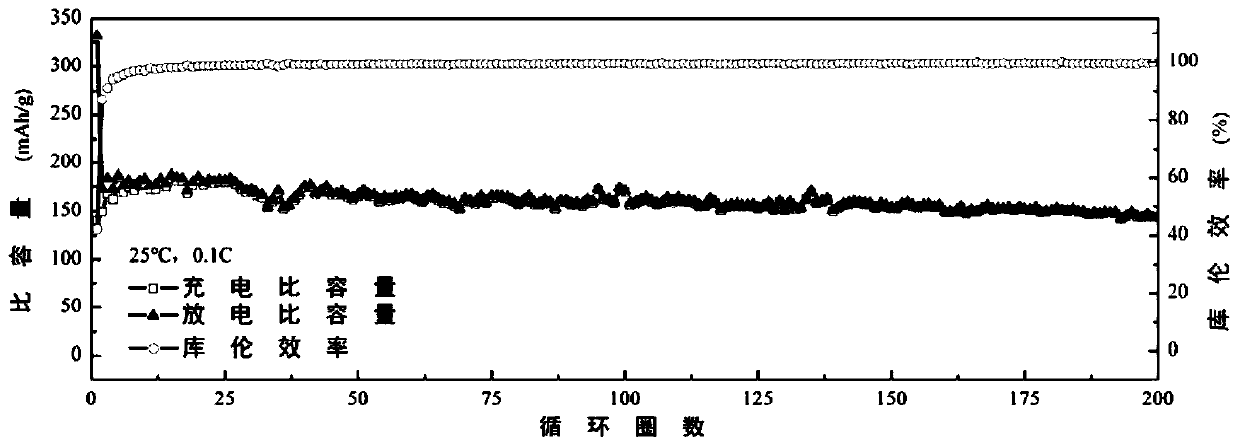
[0057] This embodiment utilizes double imidazole ring functional ionic liquid Im 2 -TFSI 2 As a functional additive of conventional imidazole-type ionic liquid EMIm-TFSI (1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide), it can not only improve the stability of the ionic liquid electrolyte, but also reduce the Increase the viscosity, increase the conductivity, and play a synergistic role among multiple ionic liquids. Specifically, the preparation method of the multiple ionic liquid electrolyte and graphite battery involved in this embodiment is as follows:
[0058] In an argon glove box, mix 1mL EMIm-TFSI with 100uL, 300uL, 500uL, 800uL and 1mL Im 2 -TFSI 2 Mix ...
Embodiment 3
[0060] Embodiment 3: Biimidazole ring functional ionic liquid Im 2 -TFSI 2 and gel polymer electrolytes and LiFePO 4 half cell
[0061] The double imidazole ring functional ionic liquid Im involved in the present embodiment 2 -TFSI 2 The preparation method is with embodiment 1.
[0062] The gel polymer electrolyte involved in this embodiment contains 26wt% PVDF-co-HFP and 74wt% (Im 2 -TFSI 2 +1mol / kg vs. m ILs LiTFSI), the preparation process is as follows:
[0063] (1) Synthesis of Biimidazole Ring Functional Ionic Liquid Im 2 -TFSI 2 : same as synthetic steps in embodiment 1;
[0064] (2) Prepare the solution: weigh 0.73g PVDF-co-HFP in an argon atmosphere glove box and dissolve it in 15mL ethylene glycol dimethanol (DME). After the dissolution is complete, add 1.6g Im 2 -TFSI 2 and 0.46g LiTFSI, magnetically stirred for 12h;
[0065] (3) Solvent volatilization: Take 5mL of the above solution and pour it into a 5cm×7cm polytetrafluoroethylene mold, first volati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrochemical stability window | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com