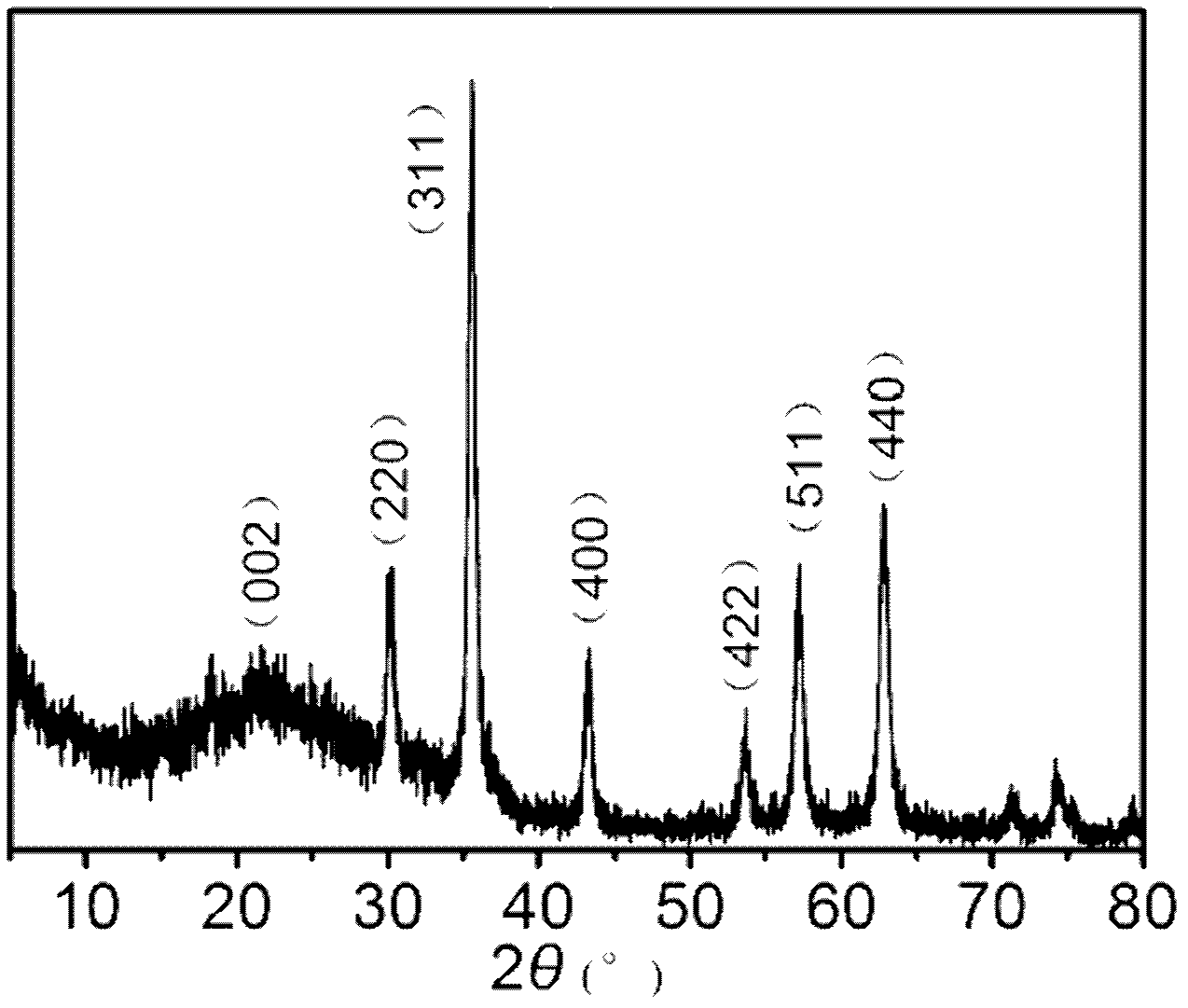
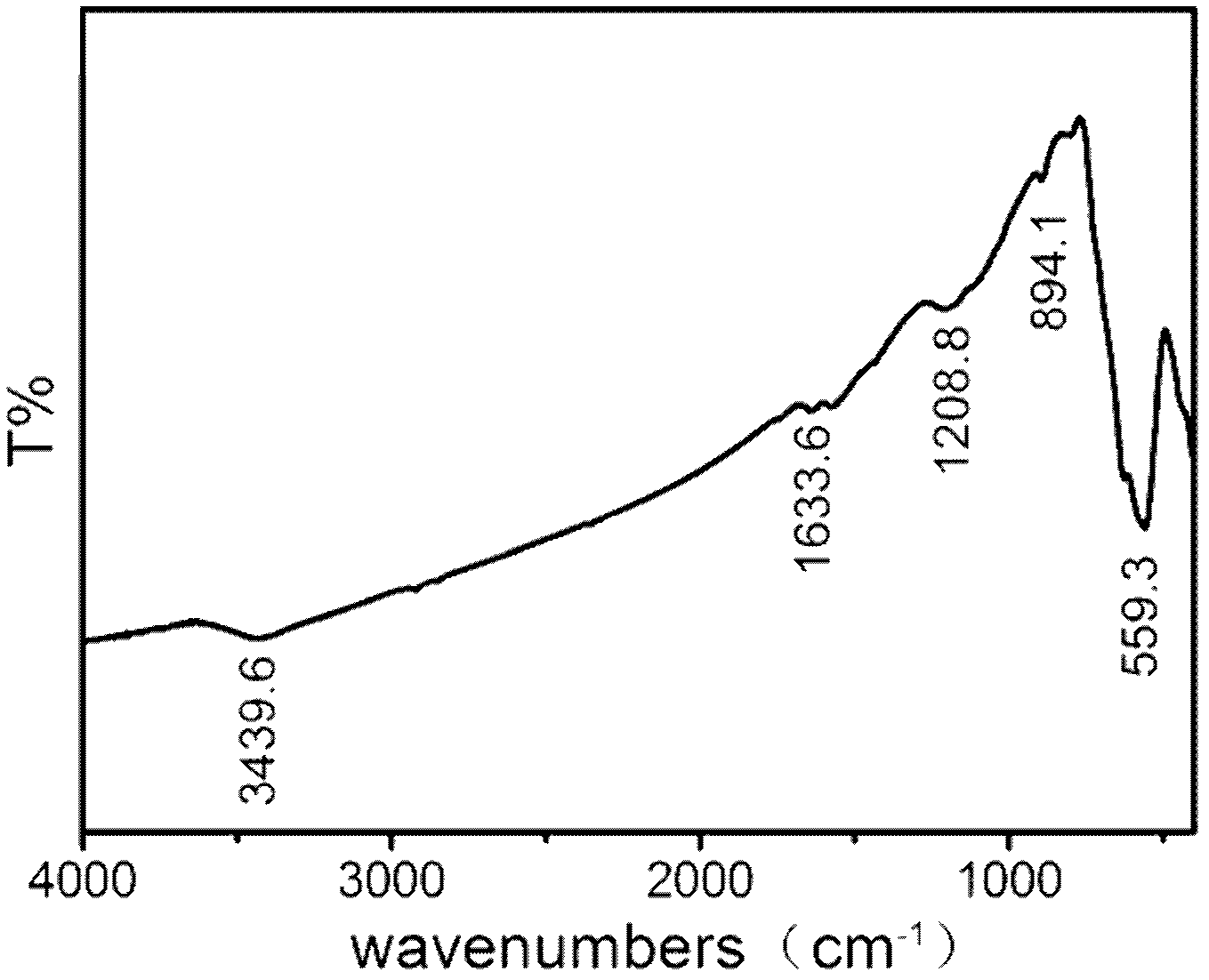
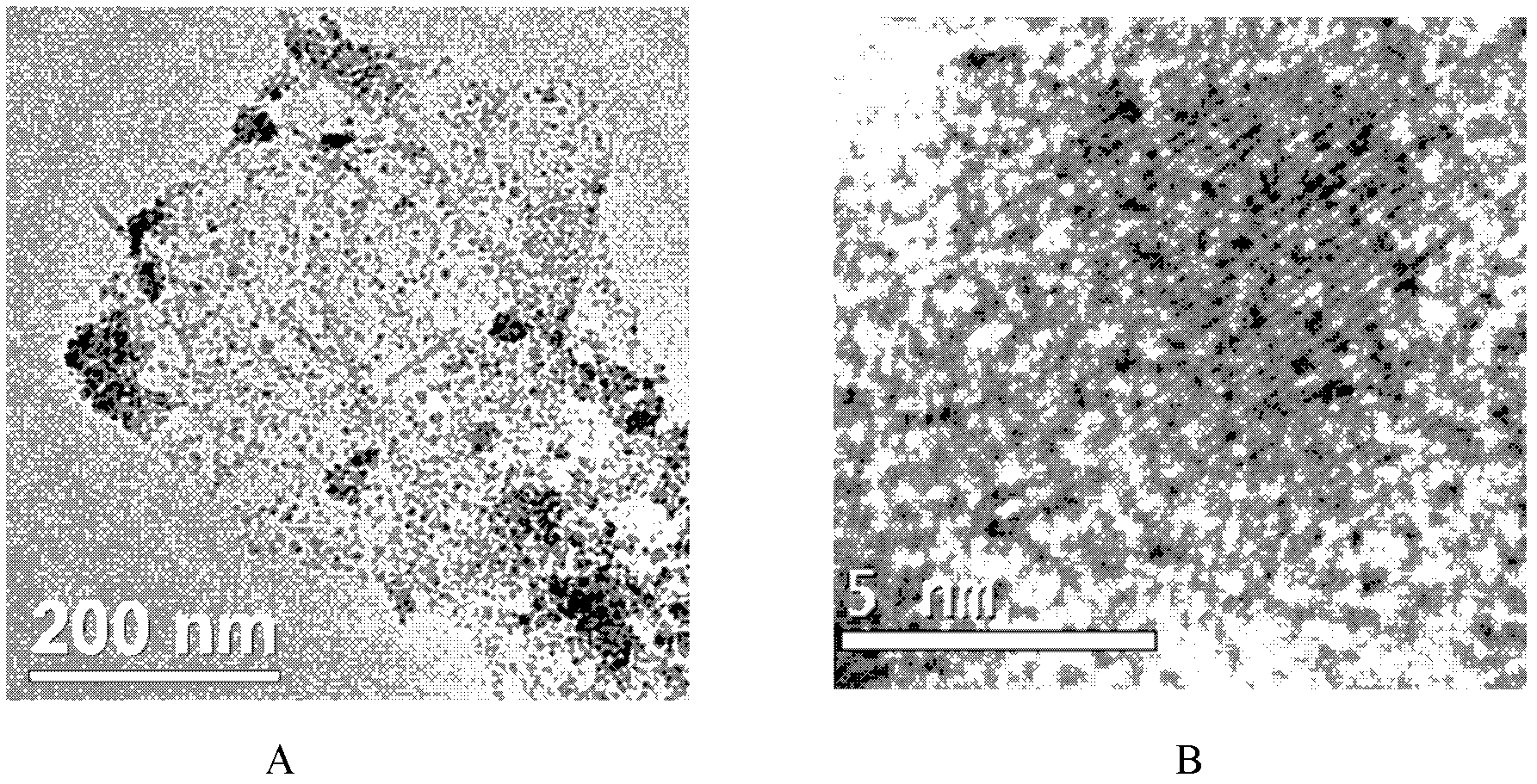
Method for preparing magnetic nanometer ferroferric oxide-graphene composite catalyst
A composite technology of ferroferric oxide and graphene, which is applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as complex preparation methods, and achieve technological Simple, avoid extra use, and improve the effect of adsorption performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the preparation method of magnetic ferroferric oxide-graphene catalyst of the present invention, comprises the following steps:
[0028] 1) Preparation of graphite oxide. Graphite oxide is prepared by oxidizing graphite with strong oxidants such as nitric acid and sulfuric acid;
[0029] 2) ultrasonically disperse 20 mg of graphite oxide in 10 mL of ethanol and 10 mL of water mixture for 1 h;
[0030] 3) Stir 0.4663g of ferric chloride hexahydrate and 0.4793g of ferrous sulfate heptahydrate in 20mL of ethanol for 20min;
[0031] 4) mixing and stirring 2) the obtained system and 3) the obtained system;
[0032] 5) After heating the reaction system in 4) to 60°C, add ammonia water to adjust the pH to 9 and react for 3 hours;
[0033] 6) The product of 5) is magnetically separated, washed with deionized water, and vacuum-dried to obtain a magnetic nano-ferric oxide-graphene composite catalyst, and use it to degrade the basic dye methylene blue to measure i...
Embodiment 2
[0035] Embodiment 2: the preparation method of magnetic ferroferric oxide-graphene catalyst of the present invention, comprises the following steps:
[0036] 1) with step one in embodiment 1;
[0037] 2) ultrasonically disperse 40 mg of graphite oxide in 10 mL of ethanol and 10 mL of water mixture for 3 h;
[0038] 3) Stir 0.4663g of ferric chloride hexahydrate and 0.2396g of ferrous sulfate heptahydrate in 45mL of ethanol for 1 hour;
[0039] 4) mixing and stirring 2) the obtained system and 3) the obtained mixture;
[0040] 5) After heating the reaction system in 4) to 50°C, add ammonia water to adjust the pH to 11 and react for 4 hours;
[0041] 6) The product of 5) is magnetically separated, washed with deionized water, and vacuum-dried to obtain a magnetic nano-ferric oxide-graphene composite catalyst, and use it to degrade the basic dye methylene blue to measure its catalytic activity. The experimental results are shown in the table 1.
Embodiment 3
[0042] Embodiment 3: the preparation method of magnetic ferroferric oxide-graphene catalyst of the present invention, comprises the following steps:
[0043] 1) with step one in embodiment 1;
[0044] 2) Ultrasonic dispersion of 20 mg of graphite oxide in 2 mL of ethanol and 10 mL of water mixture for 0.5 h;
[0045] 3) Stir 0.0466g of ferric chloride hexahydrate and 0.1078g of ferrous sulfate heptahydrate in 4mL of ethanol for 10min;
[0046] 4) mixing and stirring 2) the obtained system and 3) the obtained mixture;
[0047] 5) After heating the reaction system in 4) to 90°C, add ammonia water to adjust the pH to 10 and react for 1 hour;
[0048] 7) 6) The product of 5) was subjected to magnetic separation, washed with deionized water, and vacuum-dried to obtain a magnetic nano-ferric oxide-graphene composite catalyst, and used it to degrade the basic dye methylene blue to measure its catalytic activity, the experimental results See Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com