Amorphous ionically conductive metal oxides and sol gel method of preparation
a technology of ionically conductive metal oxides and sol gels, which is applied in the direction of electrical equipment, secondary cell details, non-aqueous electrolytes, etc., can solve the problems of hazardous conditions, problems such as cell performance, and certain liquids that are useful as effective electrolytes can create hazardous conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
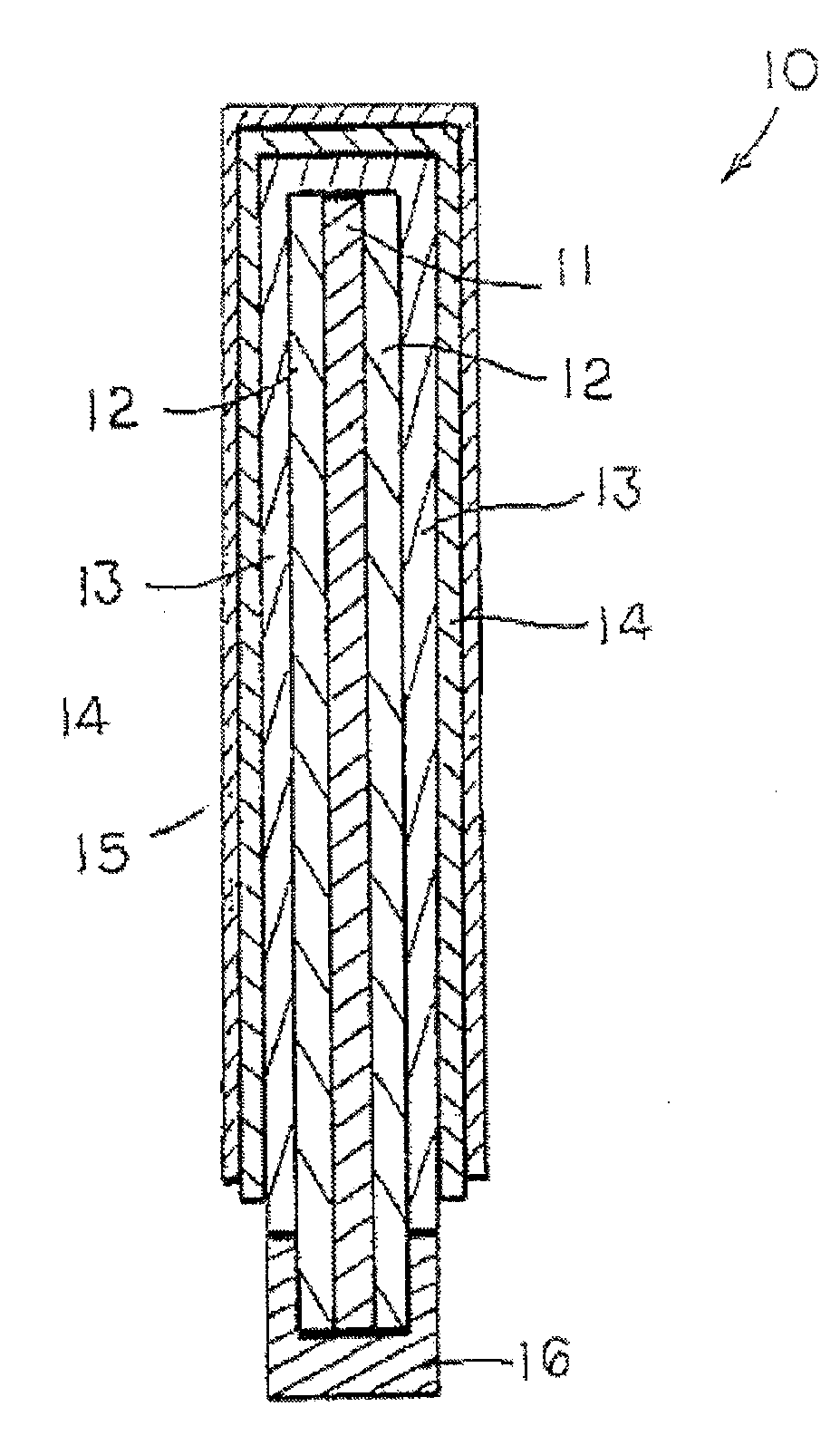
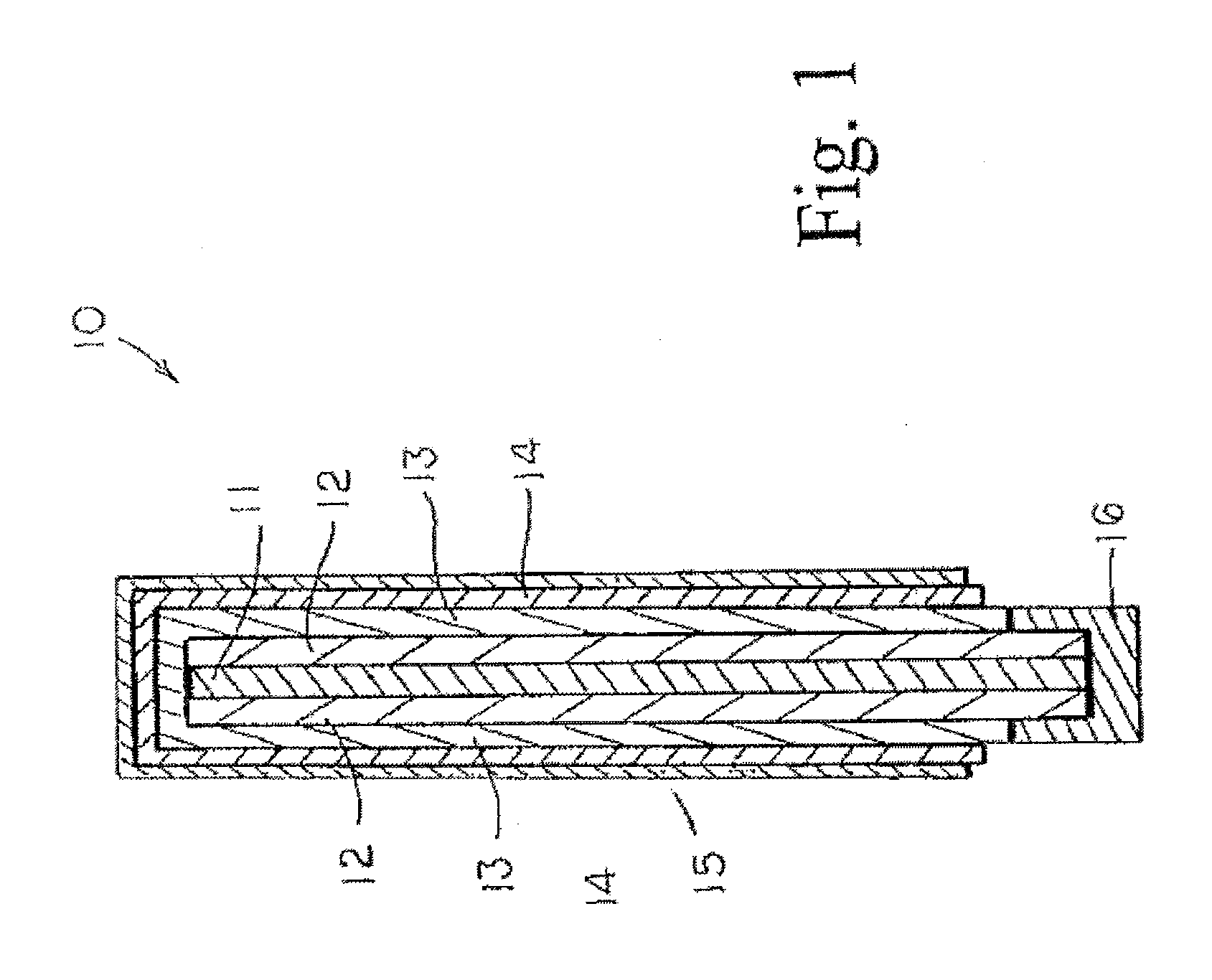
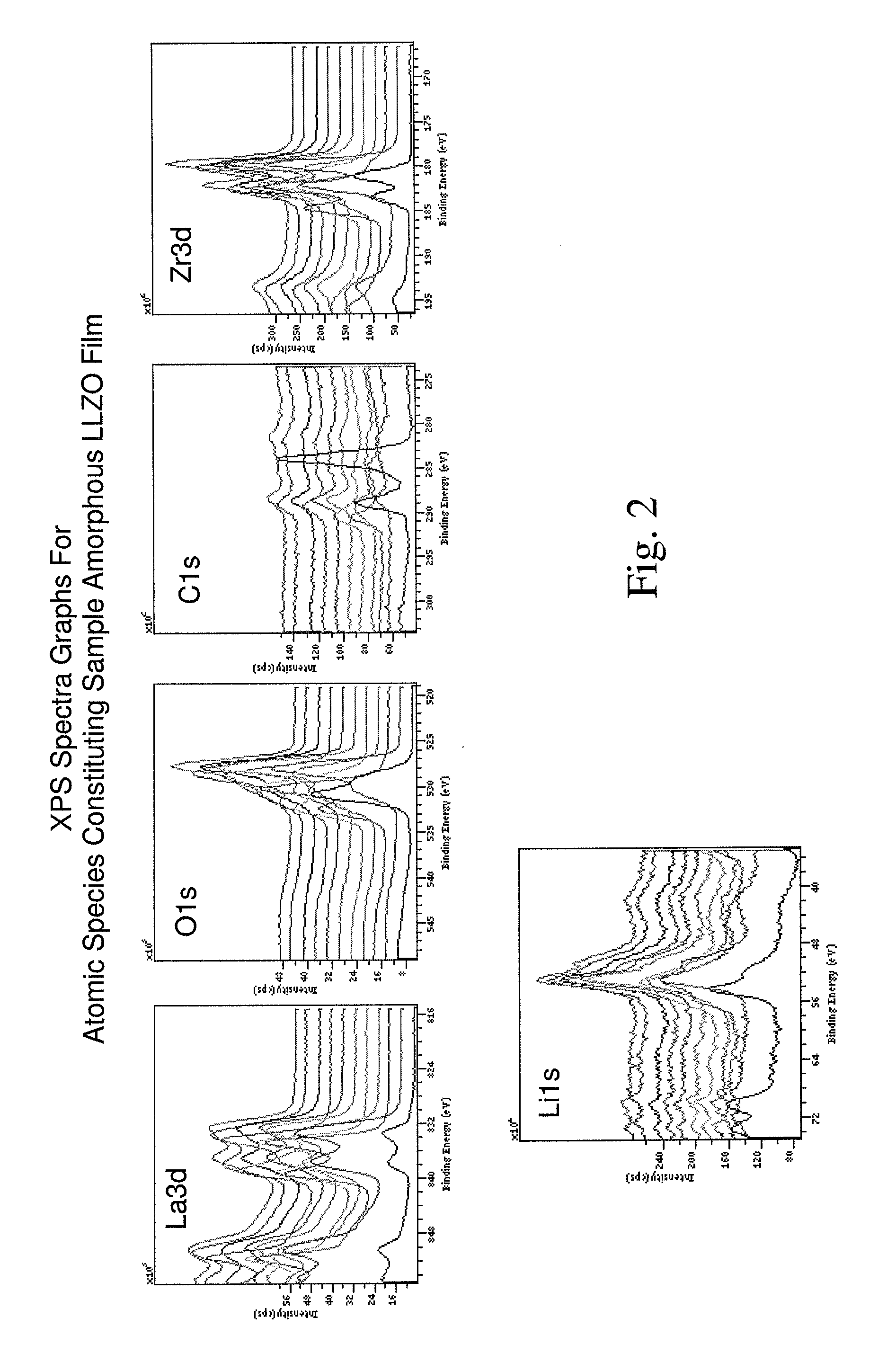
Image
Examples
synthesis examples
[0068]The ingredients in the examples described below are readily-obtainable chemical compositions that may be purchased from many different chemical suppliers in the United States such as Gelest, Inc. (Morrisville, Pa.) and Alfa Aesar (Ward Hill, Mass.).
[0069]Lithium butoxide is also know as lithium tert-butoxide (LTB); lithium t-butoxide; lithium tert-butoxide; lithium tert-butylate; 2-methyl-2-propanolithium salt; 2-methyl-2-propanol lithium salt; lithium tert-butanolate; tert-butoxylithium; tert-butylalcohol, lithium salt; lithium tert-butoxide solution; lithium butoxide min off white powder; and lithium 2-methylpropan-2-olate. It has the molecular formula C4H9LiO. It in particular may be purchased from Gelest, Inc.
[0070]Lanthanum methoxyethoxide is also known as lanthanum (III) 2-methoxyethoxide, lanthanum 2-methoxyethoxide; lanthanum methoxyethoxide; lanthanum methoxyethylate; and lanthanum tri(methoxyethoxide). It has the molecular formula C9H21LaO6. It in particular may be p...
example 1
Production of Amorphous LLZO Electrolyte Medium
[0077]An amorphous LLZO precursor solution was prepared by dissolving about 4.5 grams of a lanthanum methoxyethoxide solution, about 0.65 gram of lithium butoxide and about 0.77 gram of a zirconium butoxide solution in about 5 grams of methoxyethanol.
[0078]Lanthanum methoxyethoxide and zirconium butoxide were used in solution form for convenience in mixing. However, the invention encompasses the use of these compositions without being pre-dissolved. The lanthanum methoxyethoxide solution comprised lanthanum methoxyethoxide pre-dissolved in methoxyethanol whereby lanthanum methoxyethoxide comprised approximately 12% by weight of the total weight of the lanthanum methoxyethoxide solution. Similarly, the zirconium butoxide solution comprised zirconium butoxide pre-dissolved in butanol whereby zirconium butoxide comprised approximately 80% by weight of the zirconium butoxide solution.
[0079]The components may be mixed in any sequence, as the...
example 1 (
Example 1(B)
Formation of Film by Casting
[0084]A LLZO precursor solution described in Example 1 was optionally heated at approximately 100° C. under an inert gas to increase the density and viscosity of the solution. This optional step was utilized in some samples that were produced.
[0085]The amorphous LLZO precursor solution was cast on a suitable substrate that facilitated support and then selective release of the formed layer. The layer that was formed was initially a solution. After further processing the layer may transition into a film, or a powder, or a combination of two or more of solution, film and powder. The freshly-cast LLZO was placed in a closed container and exposed to ozone-rich air environment (ozone concentration larger than 0.05 ppm) for approximately 1 hour, although longer exposure times may be used as well. In this step, as an alternative, the closed environment may be solvent-vapor-rich (for example wherein a quantity of a solvent such as methoxyethanol is dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com