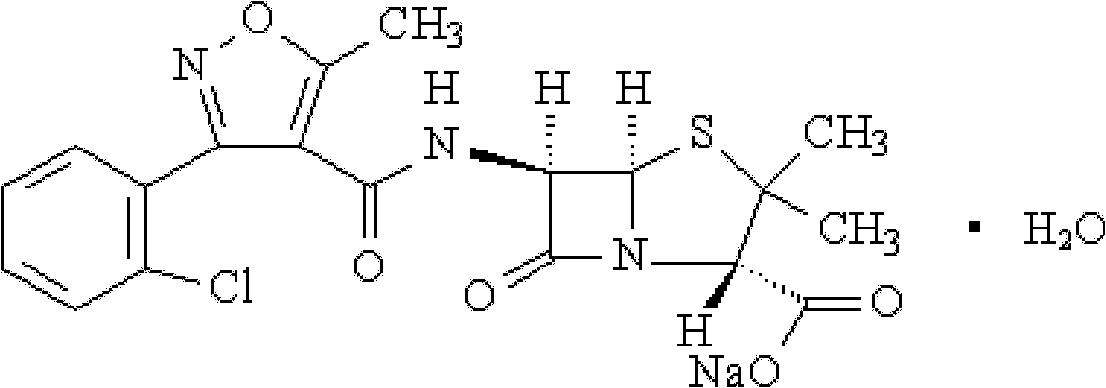
Method for crystallizing cloxacillin sodium
A cloxacillin sodium and crystallization technology, applied in the field of compound preparation, can solve the problems of uneven crystal particle size, large batch-to-batch variation, and high residual solvent, achieve uniform crystal particle size, eliminate visible foreign matter, and have good process controllability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] At room temperature, add 10g of 6-APA into 40ml of water, cool down in an ice bath, adjust the pH to 5.0-9.0 with ammonia water, and add 20ml of acetone to make solution A;
[0024] Add 11g of 3-o-chlorophenyl-5-methyl-4-isoxazole chloride into 60ml of acetone, adjust the pH of the solution to 6.0-8.0, start stirring and adjust it to 150r / min to prepare solution B.
[0025] Add 60ml of solution B into solution A, stir evenly, add a mixed solution consisting of methanol and butyl acetate with a mass ratio of 1:1; ) solution 100ml to carry out salt-forming reaction, when the generated cloxacillin sodium content reaches 10% (mg / ml), carry out vacuum distillation, vacuum pressure is-0.07~-0.09Mpa, after the crystal is separated out, carry out crystal growth 60 minutes; then perform suction filtration, wash the filter cake twice with 100 ml of acetone, and vacuum-dry at 50° C. to obtain white powder crystals (cloxacillin sodium crystals). The yield is 89.95%, the crystal gr...
Embodiment 2
[0027] At room temperature, add 10g of 6-APA into 100ml of water, cool down in an ice bath, adjust the pH to 5.0-9.0 with sodium hydroxide solution, and add 100ml of acetone to make solution A;
[0028] Add 15g of 3-o-chlorophenyl-5-methyl-4-isoxazole chloride into 100ml of acetone, and adjust the pH value of the solution to 6.0-8.0, start stirring and adjust it to 50r / min, and adjust the pH value of the solution to 6.8 -7.2, make solution B;
[0029] Add solution B into solution A, stir evenly, add a mixture of ethanol and butyl acetate with a mass ratio of 1:3; add 60 ml of butyl acetate solution of sodium isooctanoate with a mass volume ratio concentration of 30%, and perform salt formation Reaction, when the generated cloxacillin sodium content reaches 20% (mg / ml), carry out decompression distillation gradually, vacuum pressure is-0.07~-0.09Mpa, after crystal is separated out, carry out crystal growth 90 minutes; Then carry out After suction filtration, the filter cake wa...
Embodiment 3
[0031] At room temperature, add 10g of 6-APA into 75ml of water, cool down in an ice bath, adjust the pH to 5.0-9.0 with ammonia water, and add 150ml of acetone to make solution A;
[0032] Add 16g of 3-o-chlorophenyl-5-methyl-4-isoxazole chloride into 150ml of acetone, adjust the pH value of the solution to 6.0-8.0, start stirring and adjust it to 50r / min to make solution B;
[0033] Add solution B in solution A, stir evenly, add the mixed solution of isopropanol and butyl acetate that the mass ratio is 1:9; Add the sodium isooctanoate methyl isobutyl ketone (MIBK ) solution 60ml, carry out salt-forming reaction, when the generated cloxacillin sodium content reaches 20% (mg / ml), carry out decompression distillation gradually, vacuum pressure is-0.07~-0.09Mpa, after crystal is separated out, Carry out crystal growth for 120 minutes; then carry out suction filtration, wash the filter cake twice with 150ml acetone, and vacuumize and dry at 50°C to obtain white powder crystals (c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com