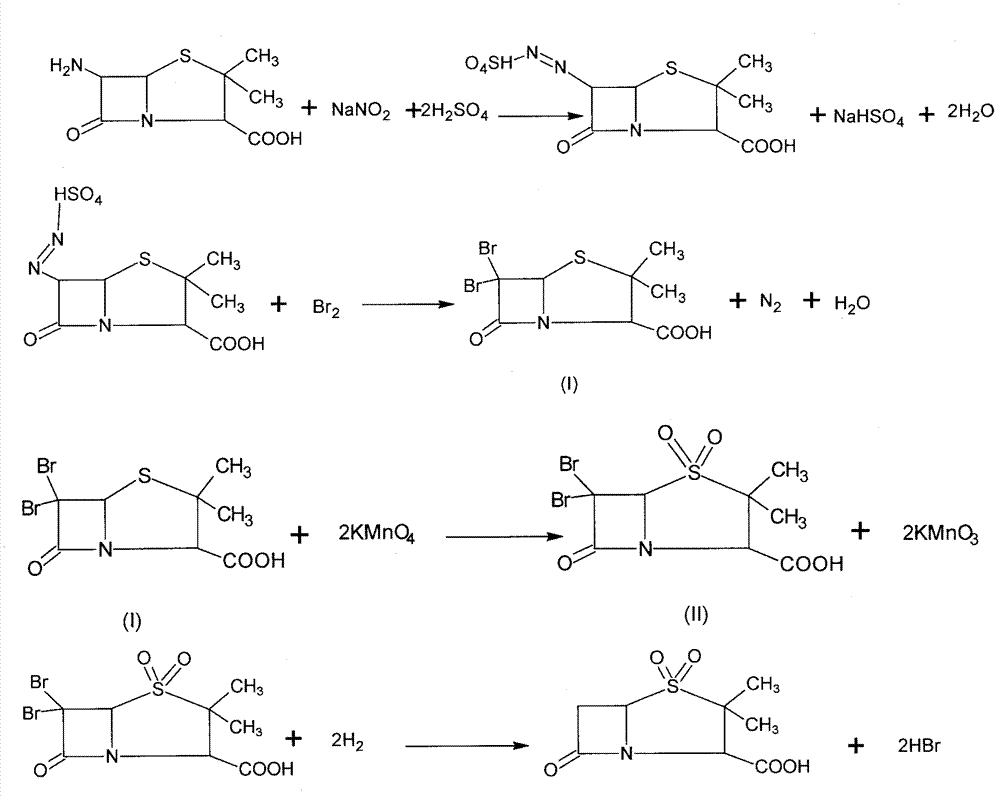
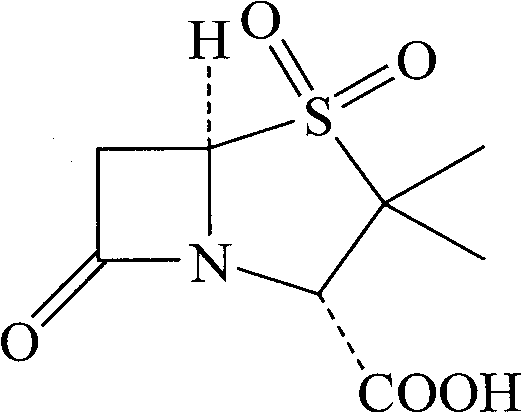
Synthesizing method of sulbactam acid
A synthesis method and technology of sulbactam acid, applied in the field of synthesis of sulbactam acid, can solve the problems of high cost, low yield, pollute the environment and the like, and achieve the effects of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Add 1000kg of ethyl acetate into a 1500L reaction pot, then stir and cool to 0°C, inhale 150kg of bromine, add sulfuric acid solution dropwise (take 43.2kg of sulfuric acid + 200kg of water), control the temperature not to exceed 5°C, cool to 4°C, Add 57.6kg of sodium nitrite in several times, control the temperature not to exceed 5°C, keep warm for 20 minutes, then slowly add 90kg of 6-APA at 4°C, and react at 10°C for 0.5 hours after the addition is complete. Add dropwise 10% sodium bisulfite aqueous solution until the bromine color disappears, and the reaction solution is light yellow. Stop stirring when no yellow gas escapes, let stand to separate layers, and extract the lower aqueous layer twice with ethyl acetate (400kg×2 ), the water layer after the extraction was recovered bromine, the ethyl acetate layer was combined to the neutralization pot and stirred and cooled, the pH was adjusted to 7 with sodium hydroxide solution, the layers were static, and the ethyl...
Embodiment 2
[0028] 1. Add 1000kg of ethyl acetate into a 1500L reaction pot, then stir and cool to 0°C, inhale 150kg of bromine, add sulfuric acid solution dropwise (take 43.2kg of sulfuric acid + 200kg of water), control the temperature not to exceed 5°C, cool to 4°C, Add 57.6kg of sodium nitrite in several times, control the temperature not to exceed 5°C, keep warm for 30 minutes, then slowly add 90kg of 6-APA at 8°C, and react at 10°C for 40 minutes after the addition is complete. Add dropwise 10% sodium bisulfite aqueous solution until the bromine color disappears, and the reaction solution is light yellow. Stop stirring when no yellow gas escapes, let stand to separate layers, and extract the lower aqueous layer twice with ethyl acetate (400kg×2 ), the water layer after the extraction is recovered bromine, the ethyl acetate layer is combined to the neutralization pot and stirred and cooled, the pH is adjusted to 7 with sodium hydroxide solution, the layers are static, the ethyl acetat...
Embodiment 3
[0033] 1. Add 1000kg of ethyl acetate into a 1500L reaction pot, then stir and cool to 0°C, inhale 150kg of bromine, add sulfuric acid solution dropwise (take 43.2kg of sulfuric acid + 200kg of water), control the temperature not to exceed 5°C, cool to 4°C, Add 57.6kg of sodium nitrite in several times, control the temperature not to exceed 5°C, keep warm for 30 minutes, then slowly add 90kg of 6-APA at 10°C, and react at 10°C for 0.4 hours after the addition is completed. Add dropwise 10% sodium bisulfite aqueous solution until the bromine color disappears, and the reaction solution is light yellow. Stop stirring when no yellow gas escapes, let stand to separate layers, and extract the lower aqueous layer twice with ethyl acetate (400kg×2 ), the water layer after the extraction is recovered bromine, the ethyl acetate layer is combined to the neutralization pot and stirred and cooled, the pH is adjusted to 7 with sodium hydroxide solution, the layers are static, the ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com