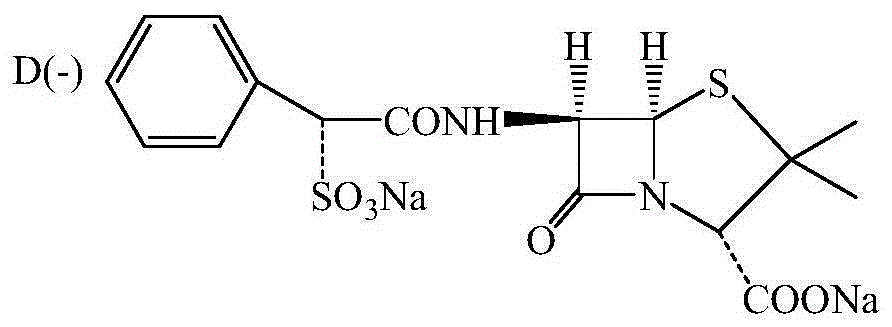
Preparing method for D(-)-sulbenicillin disodium
A technology for sulfobenicillin sodium and sulfobenicillin is applied in the field of drug synthesis, which can solve the problems of complicated purification process, long reaction steps, unsuitable industrial production and the like, and achieves improved product purity, mild reaction conditions, and no sulfur dioxide pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Preparation of dichloroethane solution of D(-)-sulfophenylacetyl chloride
[0027] Add 26.2g (0.121mol) D(-)-sulfophenylacetic acid, 78.6g solvent dichloroethane and 0.439g (0.006mol) catalyst dimethylformamide in the reaction vessel, slowly add 18.6ml2mol at 0°C / LBTC / C 2 h 4 Cl 2 Solution, after addition, reacted at room temperature for 1.5 hours, recovered the solvent by distillation under reduced pressure and removed hydrogen chloride gas to obtain 26.57g of D(-)-sulfophenylacetyl chloride, the HPLC purity was 97.75%, and the yield was 91.5%, and then 26.57g was added Dichloroethane, prepared as a dichloroethane solution of D(-)-sulfophenylacetyl chloride with a mass fraction of 50%.
[0028] (2) Preparation of D(-)-Sulbenicillin Sodium
[0029] Add 41g acetone, 20.5g75% ethanol and 20.5g (0.095mol) 6-aminopenicillanic acid in the reaction vessel, add trimethylamine until the pH of the reaction system is 7 at 10°C, stir until the 6-aminopenicillanic acid is ...
Embodiment 2-3D
[0030] The dichloroethane solution preparation of embodiment 2-3D (-)-sulfophenylacetyl chloride
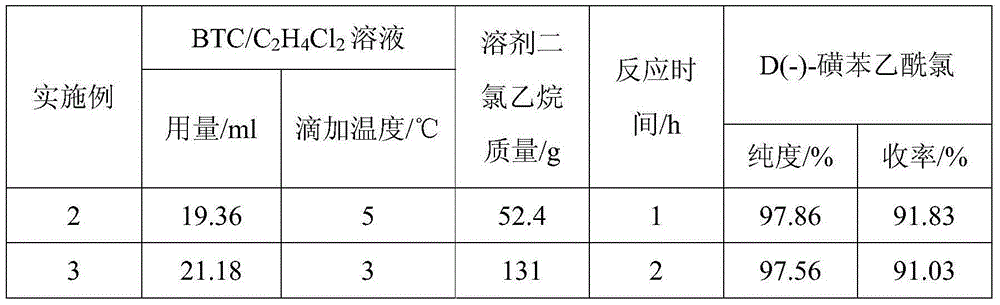
[0031] Adopt the same operating method as Example 1 (1) to prepare the dichloroethane solution of D(-)-sulfophenylacetyl chloride, the difference is BTC / C 2 h 4 Cl 2 Amount of solution and solvent dichloroethane, BTC / C 2 h 4 Cl 2 The dripping temperature of solution, reaction time, gained experimental result is shown in Table 1:
[0032] Table 1:
[0033]
Embodiment 4-5D
[0034] The preparation of embodiment 4-5D (-)-sulfbenicillin sodium
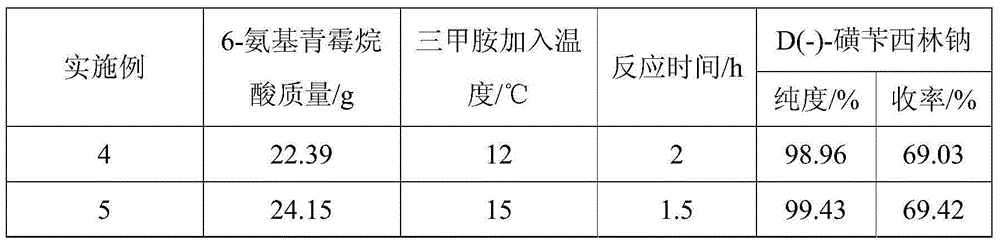
[0035] Adopt the same operating method as Example 1 (1) to prepare D (-)-sulfabenicillin sodium, the difference is the amount of 6-aminopenicillanic acid, trimethylamine adding temperature and reaction time, the experimental results obtained are shown in Table 2:
[0036] Table 2:
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com