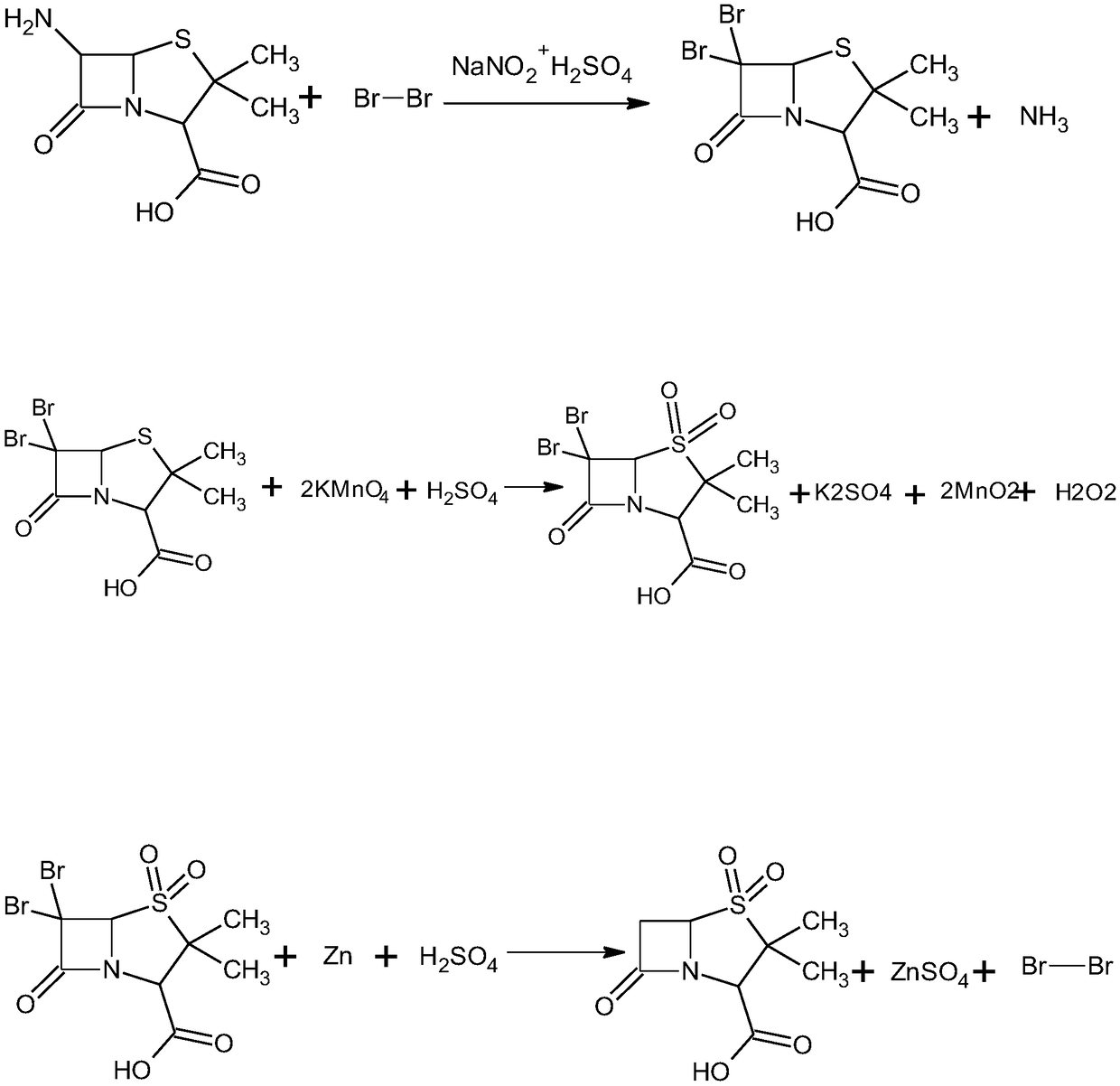
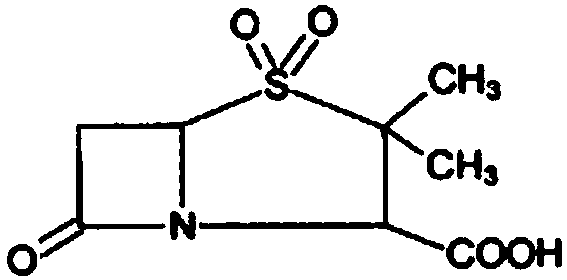
Synthesis method of sulbactam acid
A technology of sulbactam acid and a synthesis method, applied in the field of sulbactam acid synthesis, can solve problems such as low yield, and achieve the effects of improving total yield, reducing product loss, and reducing side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A kind of synthetic method of sulbactam acid, comprises the following steps:
[0043] S1, diazotization and bromination reactions, as follows:
[0044] Add 843g of ethyl acetate to No. 1 reactor, cool down to 0°C, stir at a speed of 50r / min, add 240g (1.5mol) of bromine and 420g of pure water dropwise while stirring, control the temperature at 3°C, and then 490 g (1 mol) of 20% dilute sulfuric acid was added dropwise.
[0045] Control the temperature at 4°C, stir at a speed of 250r / min, add 103.5g of solid sodium nitrite while stirring, add the solid sodium nitrite in 5 times, and add the time for 15 minutes.
[0046]Add 216g (1mol) of 6-aminopenicillanic acid into the No. 1 reaction kettle from the feeding port with a vacuum feeder, control the temperature at 25°C, keep it warm for 60min, and the reaction ends.
[0047] Add sodium bisulfite solution (750g water+375g sodium bisulfite) dropwise to No. 1 reactor, and control the temperature to 10°C to make the color of ...
Embodiment 2
[0070] A kind of synthetic method of sulbactam acid, comprises the following steps:
[0071] S1, diazotization and bromination reactions, as follows:
[0072] Add 952g of ethyl acetate to No. 1 reactor, cool down to 3°C, stir at a speed of 50r / min, add 272g (1.7mol) of bromine and 474g of pure water dropwise while stirring, control the temperature at 4°C, and then 639 g (1.5 mol) of 23% dilute sulfuric acid was added dropwise.
[0073] Control the temperature at 6°C, stir at a speed of 250r / min, add 117g of solid sodium nitrite while stirring, add the solid sodium nitrite in 5 times, and add the time for 17 minutes.
[0074] Add 216g (1mol) of 6-aminopenicillanic acid into the No. 1 reaction kettle from the feeding port with a vacuum feeder, control the temperature at 27°C, keep it warm for 60min, and the reaction ends.
[0075] Add sodium bisulfite solution (750 g of water+375 g of sodium bisulfite) dropwise to No. 1 reactor, and control the temperature to 13° C. to fade th...
Embodiment 3
[0092] A kind of synthetic method of sulbactam acid, comprises the following steps:
[0093] S1, diazotization and bromination reactions, as follows:
[0094] Add 852g of ethyl acetate to No. 1 reaction kettle, cool down to 5°C, stir at a speed of 50r / min, add bromine 320g (2mol) and pure water 430g dropwise while stirring, control the temperature at 5°C, and then drop Add 784g (2mol) of 25% dilute sulfuric acid.
[0095] Control the temperature at 8°C, stir at a speed of 250r / min, add 113.5g of solid sodium nitrite while stirring, add the solid sodium nitrite in 5 times, and add the time for 20 minutes.
[0096] Add 216g (1mol) of 6-aminopenicillanic acid into the No. 1 reaction kettle from the feeding port with a vacuum feeder, control the temperature at 30°C, keep it warm for 60min, and the reaction ends.
[0097] Add sodium bisulfite solution (750g water+375g sodium bisulfite) dropwise to No. 1 reactor, and control the temperature to 15°C to make the color of the materia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com