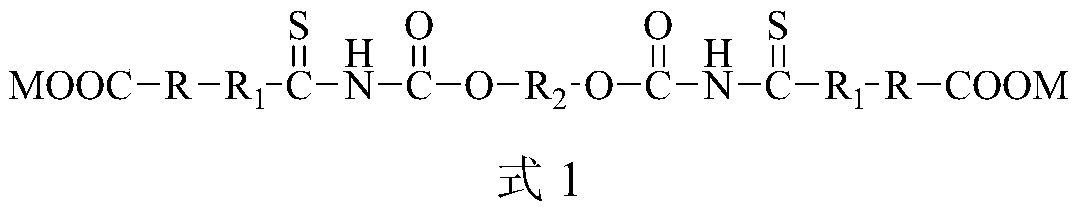
Ether-based double-sulfur amine ester derivative or ether-based bis-thiourea derivative and preparation method and application thereof
A technology of dithiourea derivatives and dithiocarbamate, which is applied in flotation, solid separation, organic chemistry, etc., to achieve the effects of small dosage, mild process conditions and improved grade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
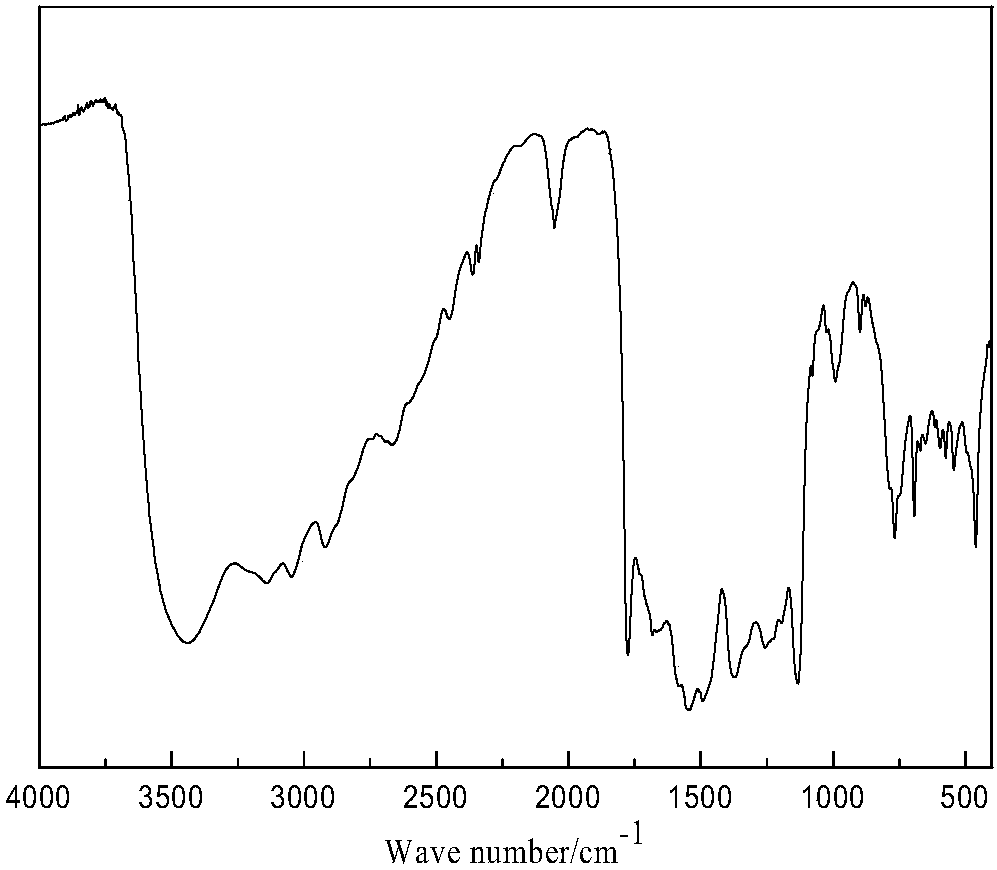
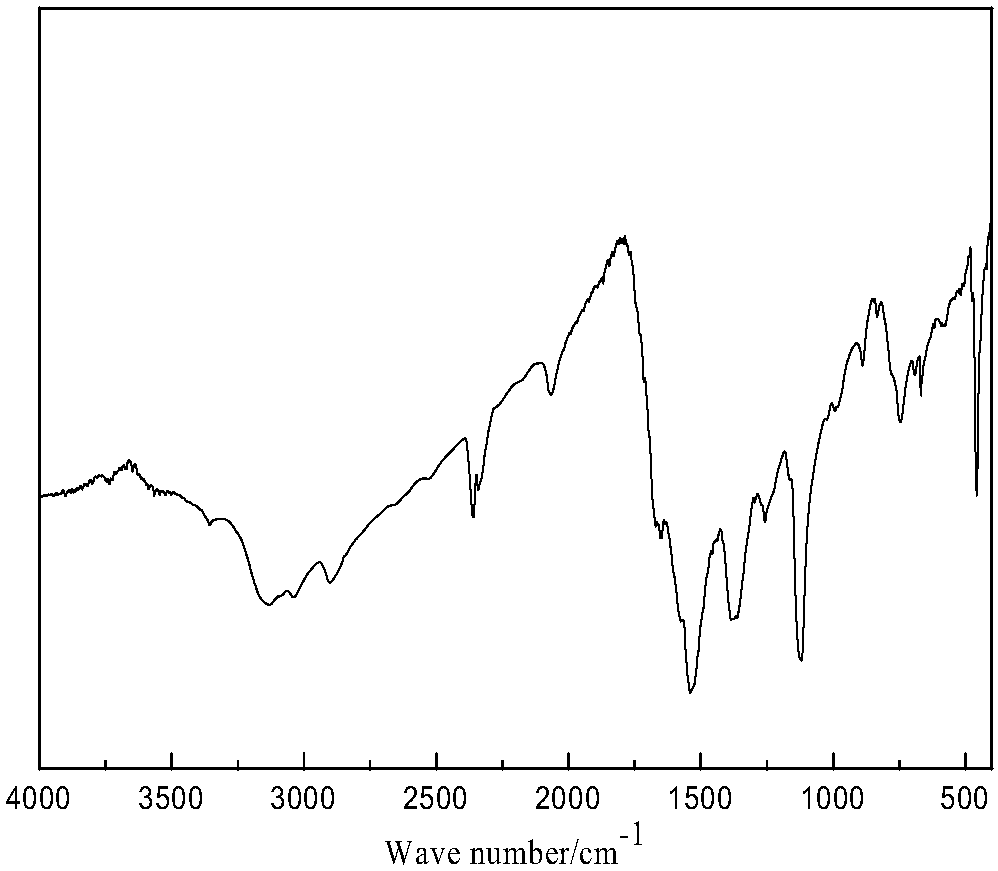
Image
Examples
Embodiment 1
[0051] Preparation of 1,4-butylenebis(oxycarbonylisothiocyanate)
[0052] In a 250mL three-necked flask, 1g of N,N-dimethylaniline, 20.25g of sodium thiocyanate (0.25mol) and 100mL of water were added dropwise with stirring 27g (0.125mol) of butylene dichloroformate, and the reaction temperature was controlled at 0-5°C, react at this temperature for 2 hours, raise the temperature to 20°C to continue the reaction for 1 hour, and separate the layers for 1 hour. The mixture is separated into an aqueous phase and an organic phase. carbonyl isothiocyanate). The yield was calculated according to diethyl dichloroformate, and the yield was 85%.
Embodiment 2
[0054] Preparation of 2,2'-Ethylene ether bis(oxycarbonyl isothiocyanate)
[0055] In a 250mL three-necked flask, add 20.25g sodium thiocyanate (0.25mol) and 100mL dichloromethane, add 2g PEG-600 at the same time, add 29g (0.125mol) diethylene glycol bischloroformate and 100mL dichloromethane dropwise under stirring Put the solution composed of methane into the above solution, control the temperature at 0-10°C, react under stirring for 2h, raise the temperature to 25°C and continue the reaction for 2h, the orange organic phase obtained after separation of the mixture is 2,2′-ethylene ether bis(oxygen carbonyl isothiocyanate). Calculate the yield according to diethylene glycol bischloroformate, and its yield is 91.5%.
Embodiment 3
[0057] Synthesis of 2-2'-triethylene glycol (oxyisothiocyanate)
[0058] In a 250mL three-necked flask, add quinoline 2g, 20.25g sodium thiocyanate (0.25mol) and 100mL toluene, add dropwise 34.4g (0.125mol) triethylene glycol bischloroformate and 50mL of toluene solution under stirring, at 0 React at -5°C for 5 hours, heat up to 25°C and continue to react for 2 hours to obtain an orange organic phase containing 2-2'-triethylene glycol (oxyisothiocyanate), according to triethylene glycol dichloroformic acid The calculated yield of the ester was 80.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com