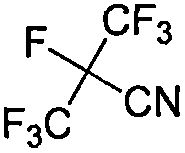
Method for synthesizing perfluoroisobutyronitrile
A technology of perfluoroisobutyryl nitrile and heptafluoroisobutyramide is applied in chemical instruments and methods, preparation of organic compounds, dehydration preparation of carboxylic acid amides, etc. Isobutyryl fluoride has no problems such as commercial supply and complex electrolytic fluorination process, and achieves the effects of easy separation, high product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
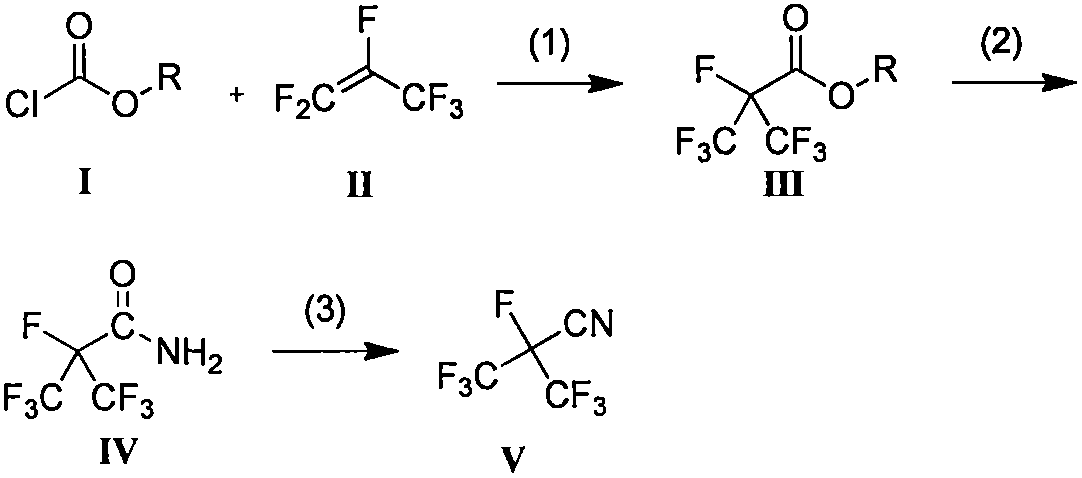
[0022] (1) Preparation of ethyl heptafluoroisobutyrate intermediate Add ethyl chloroformate 50g (460.73mmol), hexafluoropropylene 82.94g (552.88mmol), anhydrous potassium fluoride 58.89g (1.01 mol) and 300 mL of diethylene glycol dimethyl ether were stirred and reacted at 50° C. for 3 h, and the reaction mixture was distilled to obtain 95.80 g of a colorless transparent liquid product. The product purity is 90.5%, and the yield is 80.31%. 1 H NMR (500 MHz, CDCl 3 )δ4.49~4.45(m, 2H), 1.40~1.37(t, 3H); 19 F NMR (471MHz, CDCl 3 )δ -74.94~-74.96 (d, J=9.42Hz, 6F), -182.09~-182.17 (m, 1F).
[0023] (2) Preparation of heptafluoroisobutyramide intermediate Dissolve 100g (413.07mmol) of ethyl heptafluoroisobutyrate in 500mL of methanol, lower the internal temperature of the reaction kettle to -20°C, and slowly pass Add 7.03g (413.07mmol) of ammonia gas. After the feeding was completed, the temperature of the kettle was slowly raised to room temperature. After continuing the react...
Embodiment 2
[0026] (1) Preparation of methyl heptafluoroisobutyrate intermediate Add methyl chloroformate 45.00g (476.21mmol), hexafluoropropylene 142.88g (952.41mmol) and tetrabutylammonium fluoride 243.28 g ( 1.19mol) and anhydrous acetonitrile 500mL, stirred at 120°C for 10h. The resulting reaction mixture was distilled to obtain 83.90 g of a colorless transparent liquid product. The product purity is 91.34%, and the yield is 70.56%. 1 H NMR (500MHz, CDCl 3 )δ4.01(s, 3H); 19 F NMR (471MHz, CDCl 3 ) δ -75.11 ~ -75.13 (d, J=9.42Hz, 6H), -182.29 ~ -182.39 (m, 1H).
[0027] (2) Preparation of Heptafluoroisobutyramide Intermediate Add ethyl heptafluoroisobutyrate (100.00 g, 438.47 mmol) into a 250 mL dry three-necked flask. Slowly add ammonia methanol solution 125.28 mL (7M, 876.94 mmol) dropwise into the system under stirring, and control the system temperature not higher than 20°C during the addition. After the addition was completed, the reaction was continued for 2 hours, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com