Method for synthesizing Nexavar
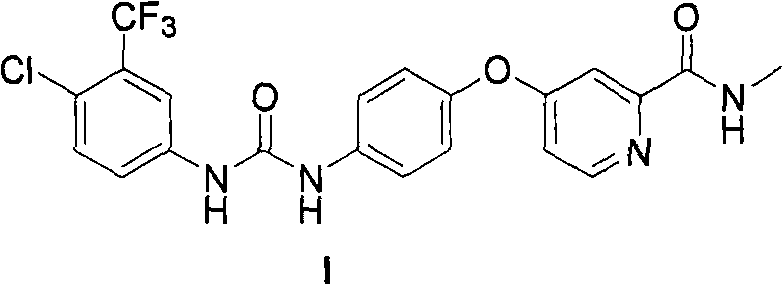
A synthesis method and compound technology, which can be applied in the fields of drug combination, antitumor drugs, organic chemistry, etc., can solve the problems of poor economy, low yield, and low compound VI synthesis yield, avoid the use of phosgene, and improve safety. and operability, improved product purity and environmental compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
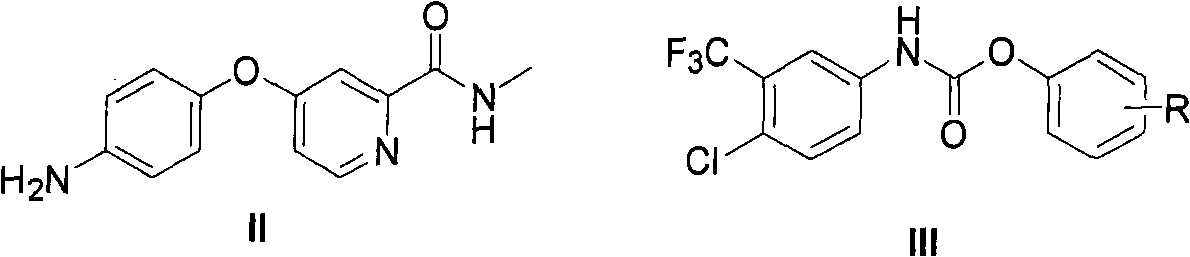
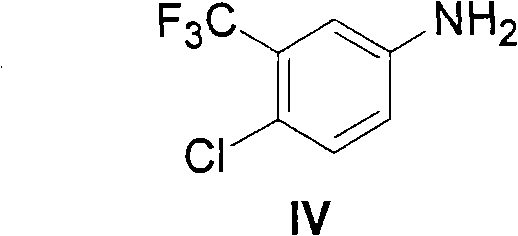
[0042] 1.1 Preparation of phenyl (4-chloro-3-trifluoromethyl-phenyl)carbamate
[0043] Add compound IV (4-chloro-3-trifluoromethyl-benzene, Gaoyou Guangming Chemical Factory, chemically pure) 5g in the there-necked flask, add 50ml of dichloromethane (Shanghai Chemical Reagent Co., Ltd., chemically pure) and stir to dissolve completely, Add 4 g of pyridine (Shanghai Chemical Reagent Co., Ltd., chemically pure). A mixed solution of 10 ml of dichloromethane and 4.2 g of phenyl chloroformate (Shanghai Chemical Reagent Co., Ltd., chemically pure) was added dropwise at room temperature, and reacted for half an hour. TLC (PE: EA = 3: 1) showed that the reaction of the raw material was complete, washed once with 1N hydrochloric acid, washed three times with water, dried, filtered, weighed 7.6 g after drying, and the molar yield was 94%.
[0044] 1 H NMR (CDCl 3 , 500MHz): δ=7.1(s, 1H), 7.2(d, J=7Hz, 2H), 7.3(m, 1H), 7.4(m, 2H), 7.5(d, J=8Hz, 1H), 7.6 (d, J=8Hz, 1H), 7.8 (s, 1H). ...
Embodiment 2
[0059] 2.1 Preparation of 4-nitrophenyl (4-chloro-3-trifluoromethyl-phenyl)carbamate
[0060] Add compound IV (4-chloro-3-trifluoromethyl-benzene, Gaoyou Guangming Chemical Factory, chemically pure) 5g in the there-necked flask, add 50ml of dichloromethane (Shanghai Chemical Reagent Co., Ltd., chemically pure) and stir to dissolve completely, Add 4 g of pyridine (Shanghai Chemical Reagent Co., Ltd., chemically pure). A mixed solution of 10 ml of dichloromethane and 5.1 g of p-nitrophenyl chloroformate (Shanghai Chemical Reagent Co., Ltd., chemically pure) was added dropwise at room temperature, and reacted for half an hour. TLC (PE: EA = 3: 1) showed that the reaction of the raw material was complete, washed once with 1N hydrochloric acid, washed three times with water, dried, filtered, weighed 8.4 g after drying, and the molar yield was 92%.
[0061] 1 H NMR (CDCl 3 , 500MHz): δ=7.1(d, J=7Hz, 1H), 7.2(dd, J=7Hz, J=3Hz, 1H), 7.3(d, J=7Hz, 2H), 7.7(d, J=3Hz , 1H), 7.8 (s, 1...
Embodiment 3
[0076] 3.1 Preparation of 2-nitrophenyl (4-chloro-3-trifluoromethyl-phenyl)carbamate
[0077] Add compound IV (4-chloro-3-trifluoromethyl-benzene, Gaoyou Guangming Chemical Factory, chemically pure) 5g in the there-necked flask, add 50ml of dichloromethane (Shanghai Chemical Reagent Co., Ltd., chemically pure) and stir to dissolve completely, Add 4 g of pyridine (Shanghai Chemical Reagent Co., Ltd., chemically pure). A mixed solution of 10 ml of dichloromethane and 5.1 g of o-nitrophenyl chloroformate (Shanghai Chemical Reagent Co., Ltd., chemically pure) was added dropwise at room temperature, and reacted for half an hour. TLC (PE: EA = 3: 1) showed that the reaction of the raw material was complete, washed once with 1N hydrochloric acid, washed three times with water, dried, filtered, weighed 8.4 g after drying, and the molar yield was 92%.
[0078] 1 H NMR (CDCl 3 , 500MHz): δ=7.1(d, J=7Hz, 1H), 7.2(dd, J=7Hz, J=3Hz, 1H), 7.3(dd, J=7Hz, J=3Hz, 1H), 7.3(m , 1H), 7.6 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com