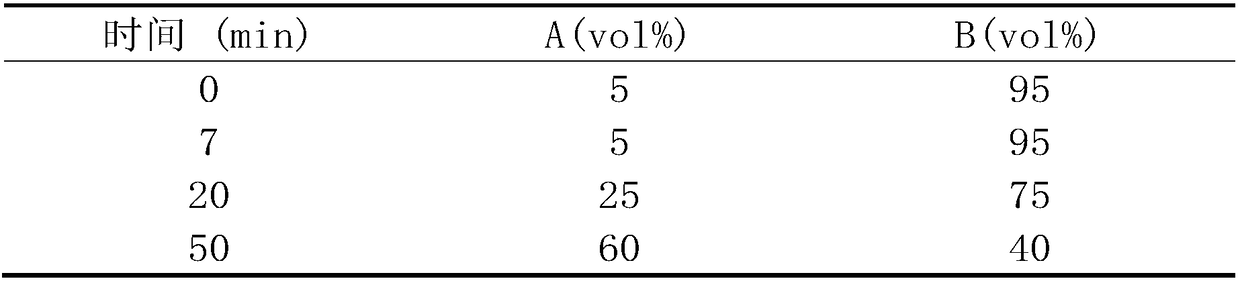
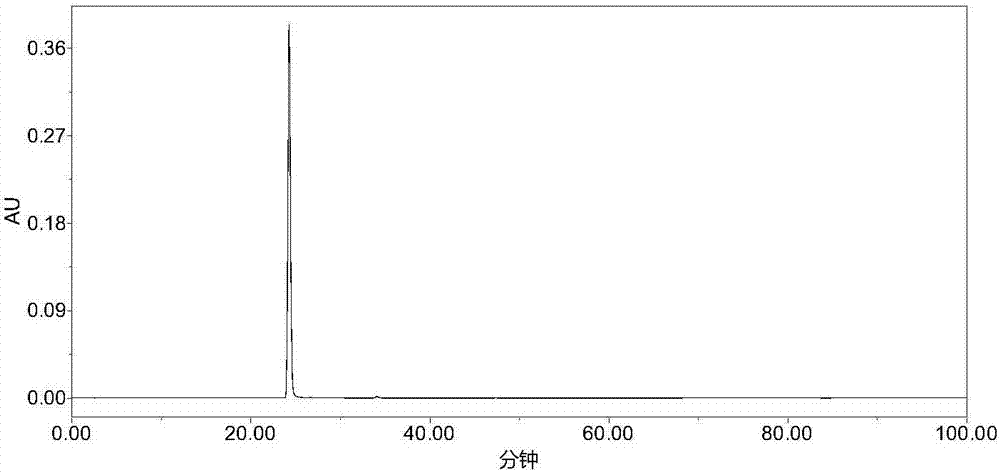
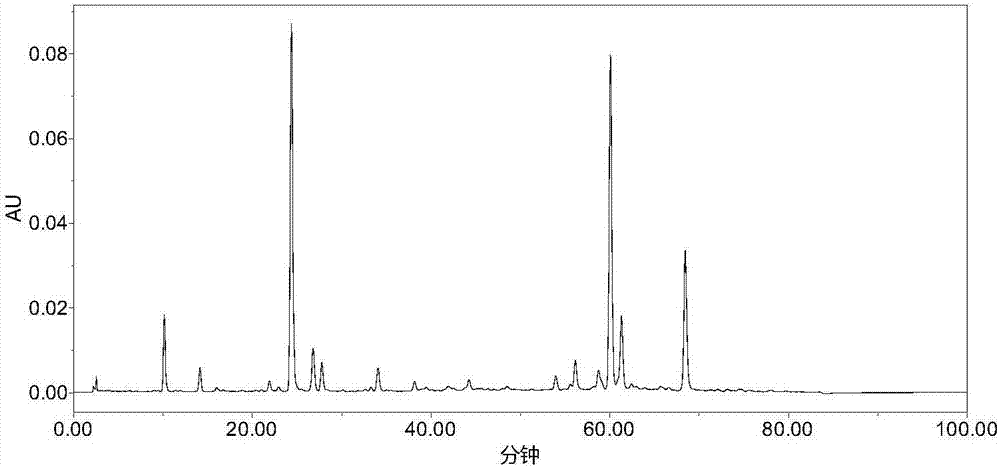
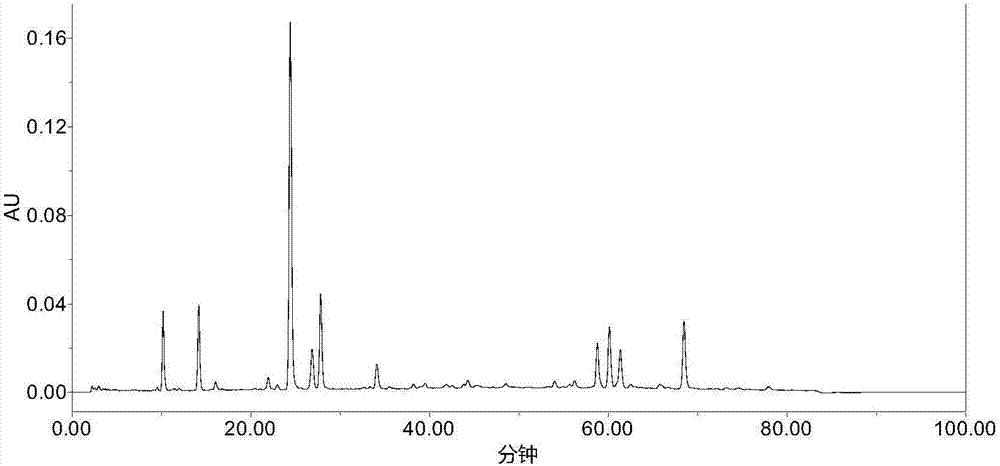
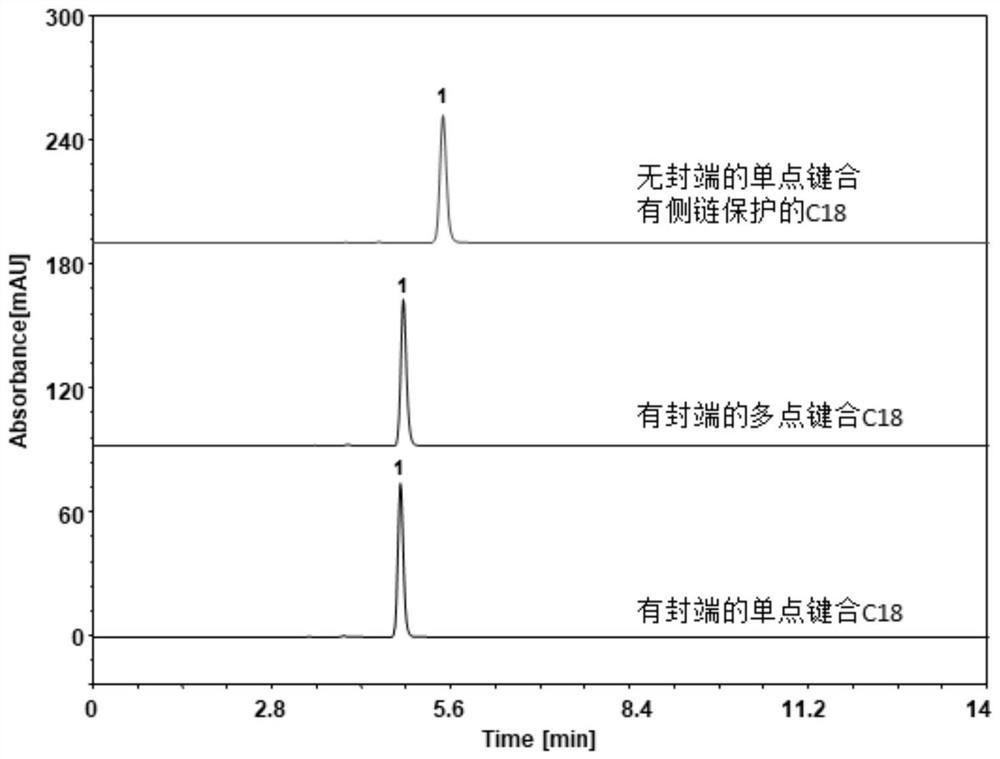
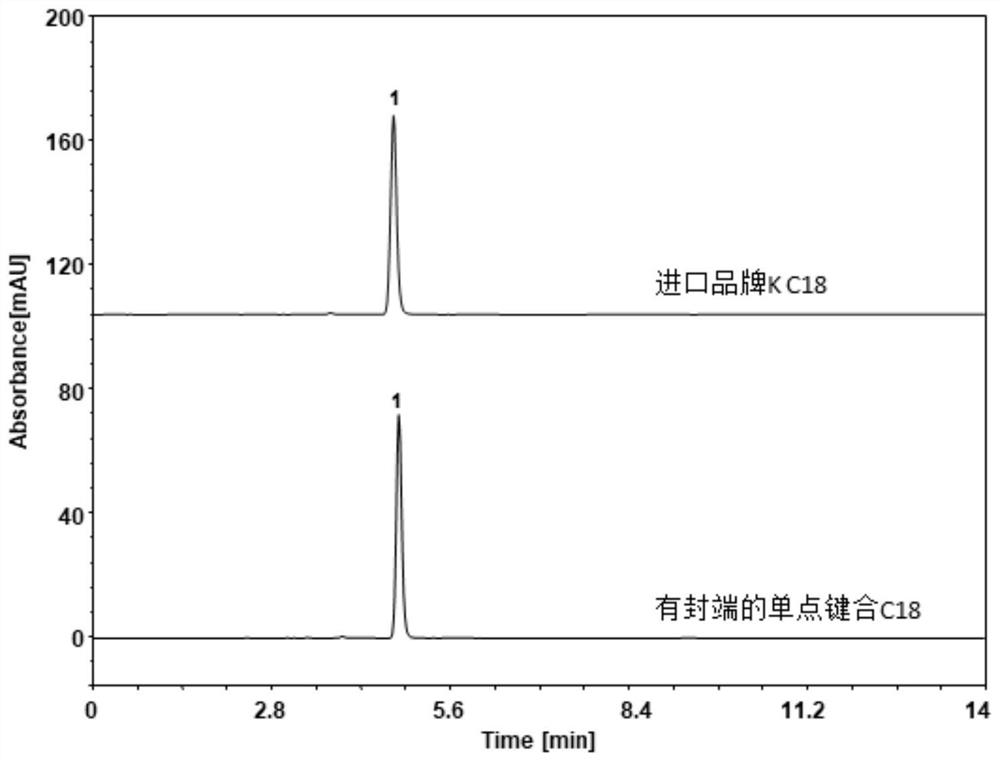
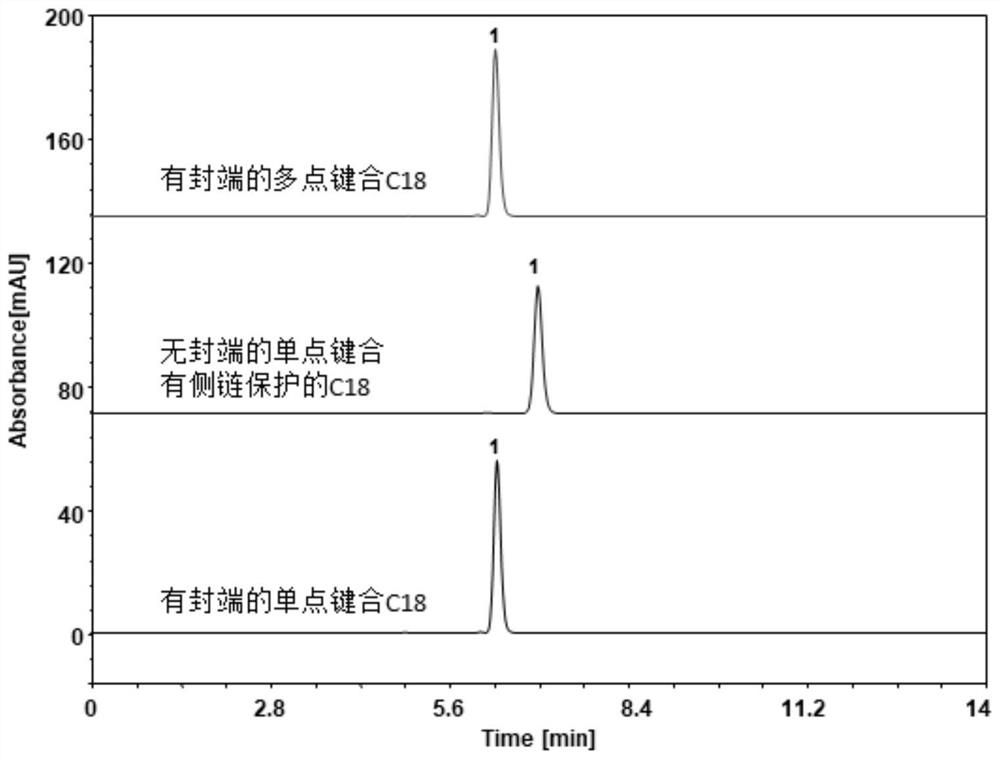
Patents
Literature
48results about How to "Stable retention time" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
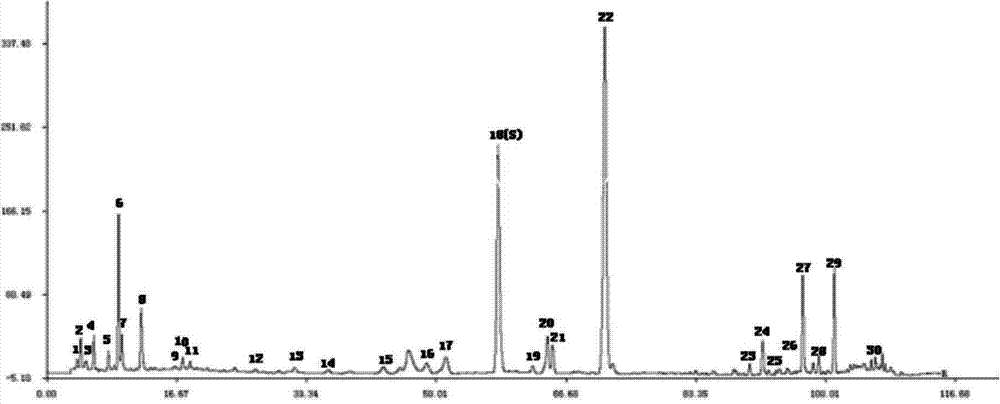
Method for determining fingerprint spectrum of traditional Chinese medicine for treating coronary heart disease
ActiveCN103760254AEffective symptomatic treatmentConducive to reflect the qualityComponent separationCoronary artery diseasePhosphoric acid
The invention relates to a method for determining fingerprint spectrum of traditional Chinese medicine for treating coronary heart disease. The method comprises the following steps: preparing a test solution, preparing a reference solution, and carrying out gradient elution under the chromatographic conditions that an Agilent ZORBAX Extend C18 chromatographic column is used as a filling agent, a mobile phase A is methanol and a mobile phase B is 0.4% phosphoric acid water solution; determining under conditions that the flow velocity is at 1.0ml / min, the column temperature is at 35 DEG C and the detection wavelength is 230-240nm, thus obtaining the fingerprint spectrum of traditional Chinese medicine. The method has the characteristics of being simple and convenient, good in reproducibility, more in characteristic peaks, accurate and reliable, and the like, and the quality of the traditional Chinese medicine preparation can be controlled effectively by the established fingerprint spectrum.
Owner:SHAANXI BUCHANG PHARMA
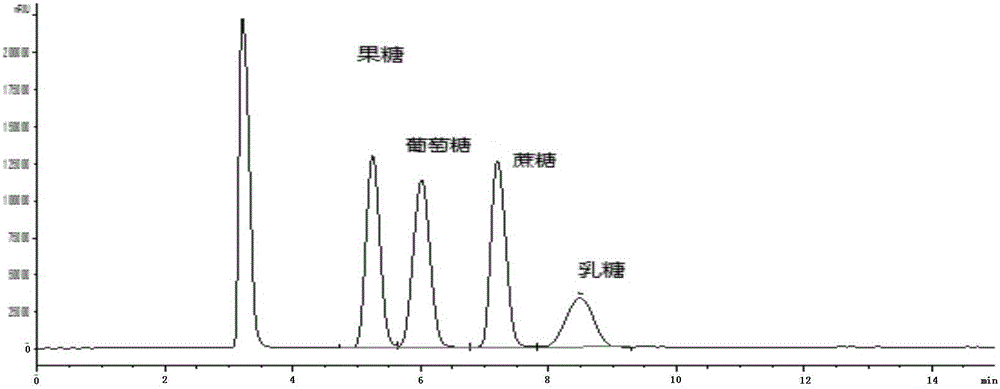
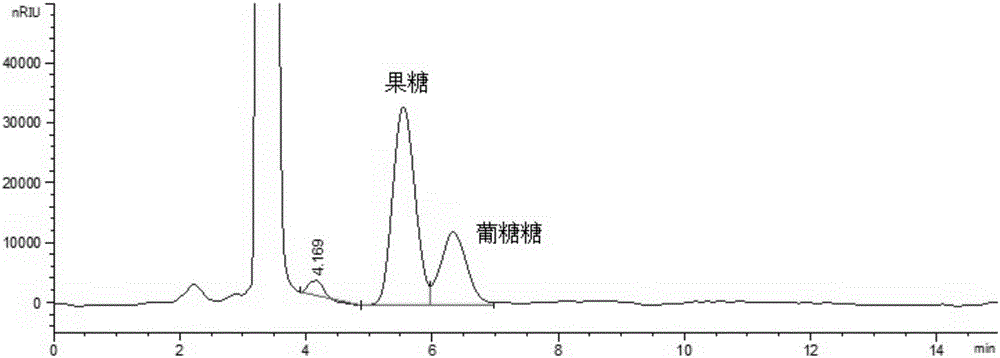
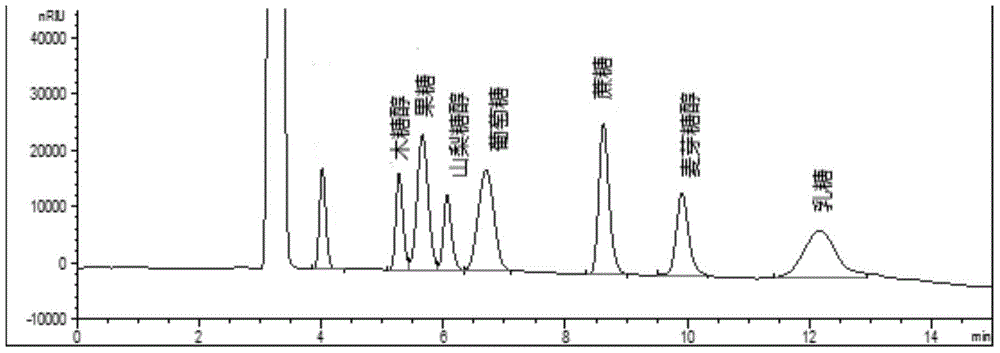
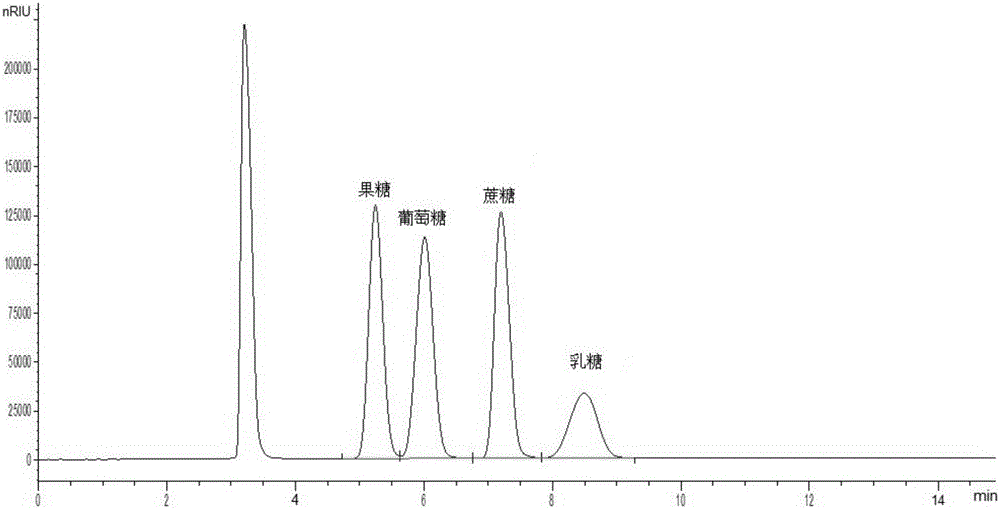
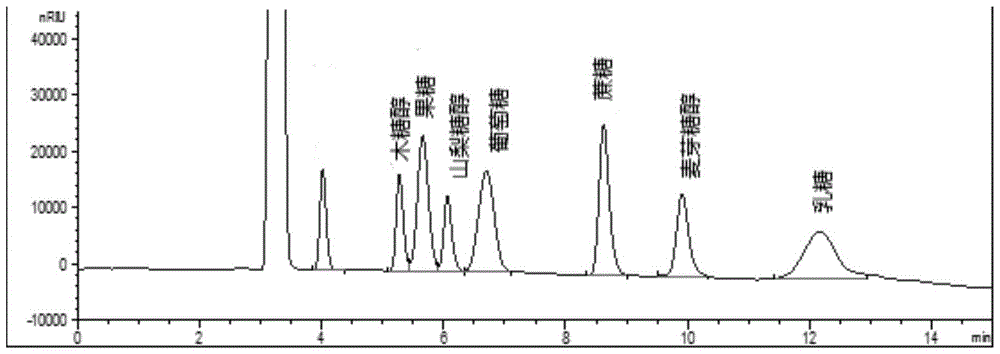
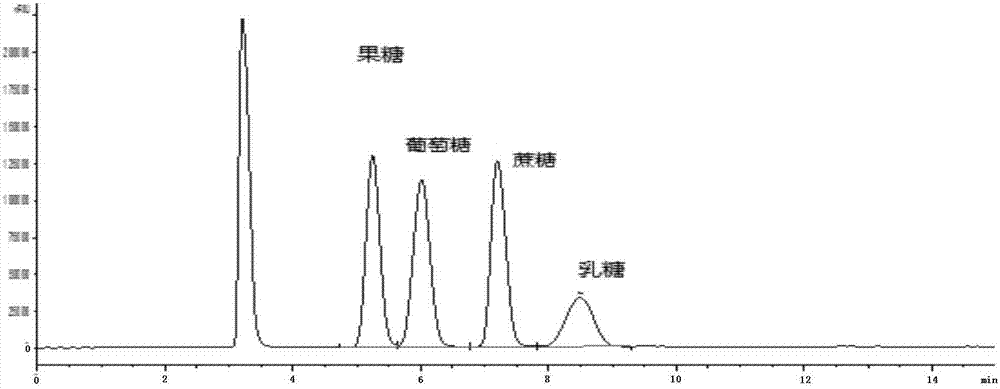
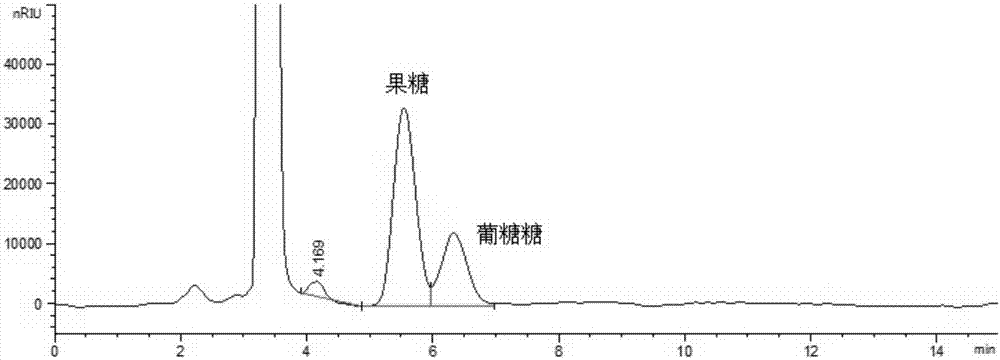
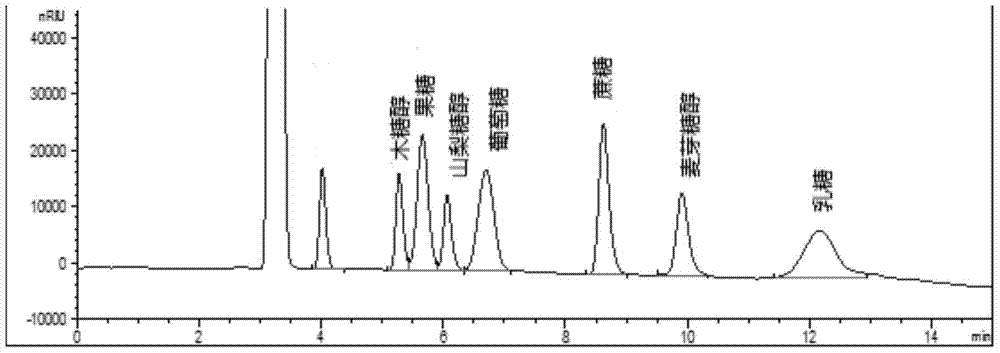
Method for simultaneously detecting multiple sugars and sugar alcohols in food
InactiveCN105717207AReduce churnImprove toleranceComponent separationAlcohol sugarsColumn temperature
The invention relates to a method for simultaneously detecting multiple sugars and sugar alcohols in food. An HPLC-differential refraction detection method is adopted as the method; the method is characterized by comprising the HPLC chromatographic conditions that an amide-bonded column is adopted as a chromatographic column, single mobile phases of acetone, water and triethylamine are adopted as mobile phases, isocratic elution is performed, the column temperature is 70 DEG C, the flowing velocity is 0.8 ml / min, and the sample injection amount is 10 milliliters. The detection method can be suitable for the food such as honey, wine and baijiu; detection can be performed only by simply processing a sample, the method is rapid, high in recovery rate, wide in linear range, good in correlation coefficient and low in detection limit, and the blank of simultaneous detection on the sugars and sugar alcohols in the food in China is filled up.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
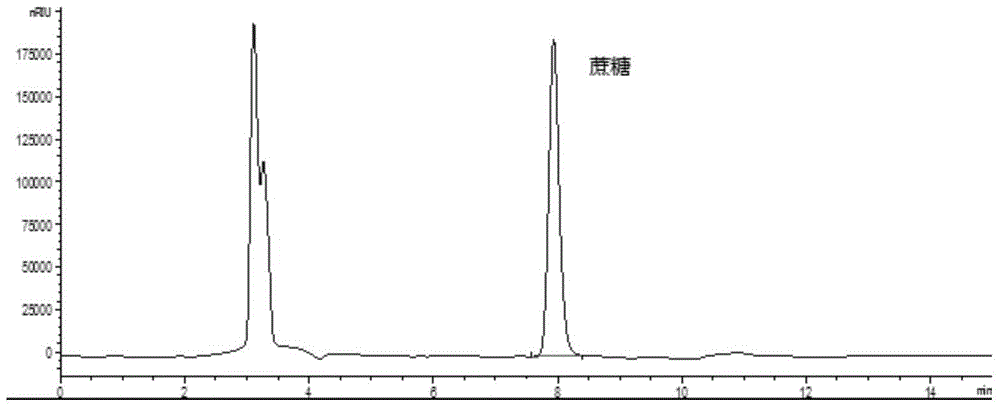
Method for determining glucose, fructose and saccharose in tobacco essence perfume
InactiveCN102520094AHigh detection sensitivityLow detection limitComponent separationChemistryChromatography column
The invention discloses a method for determining glucose, fructose and saccharose in tobacco essence perfume through a high performance liquid chromatography / differential refraction detector. The method comprises the following steps: dissolving a sample through 0.01 mol / L of caustic soda solution to be constant volume; adopting a cation exchange chromatography column, which takes highly cross-linked lead type sulfonated styrene-divinylbenzene (SDVB) resin as a filler, as a separation column; using pure water as a moving phase to carry out isogradient elution; detecting through the differential refraction detector; using an external standard method for quantification; and computing contents of the glucose, the fructose and the saccharose. The method has favorable linearity with correlation coefficient being greater than 0.999, recovery of 97.1 percent-102.4 percent, within-day precision being less than 1.2 percent, day to day precision being less than 3.0 percent, detection limit being lower than 1.8mug, and good sensitivity, and is suitable for analyzing and determining the glucose, the fructose and the saccharose for various tobacco essence perfume samples.
Owner:CHINA TOBACCO GUANGDONG IND
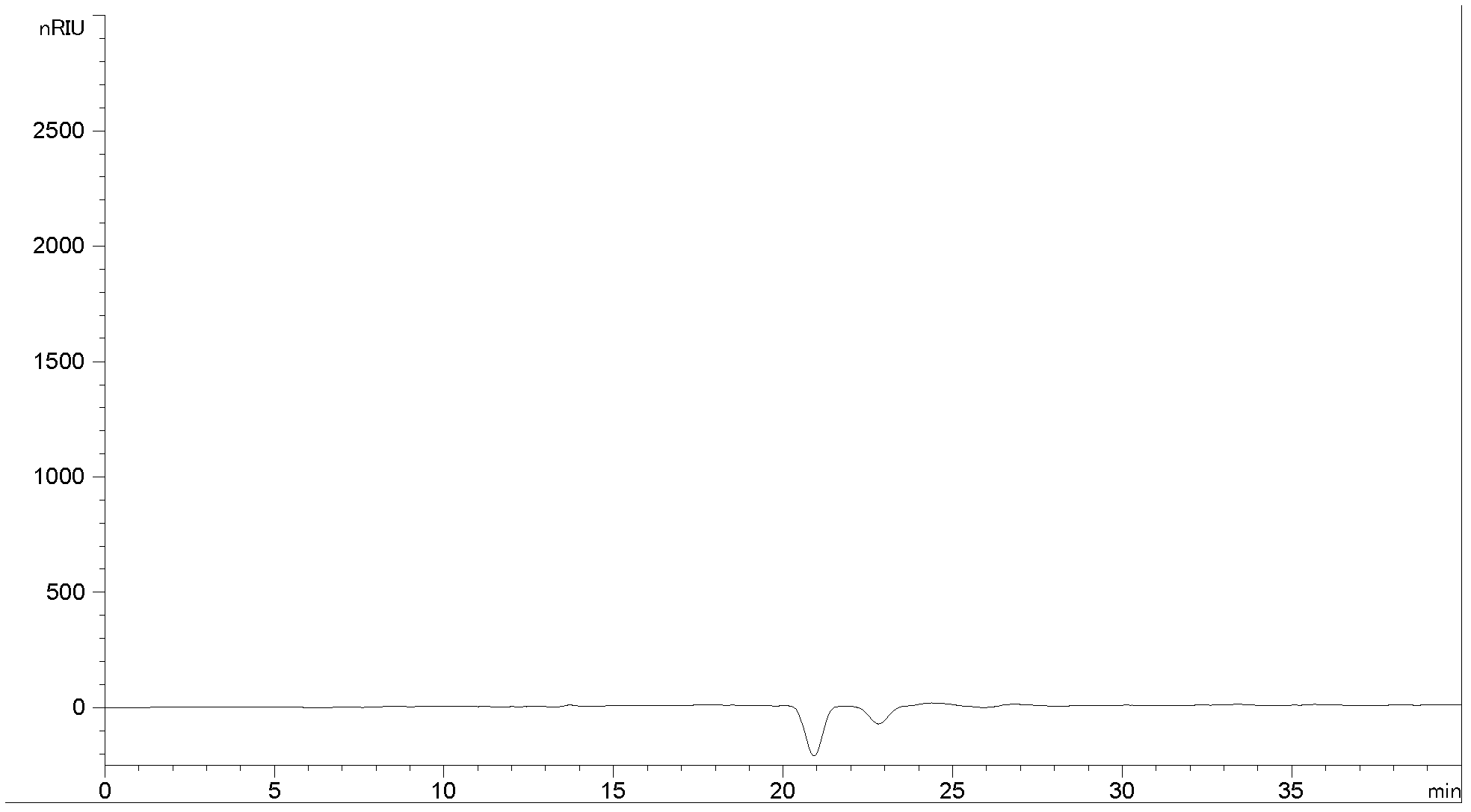
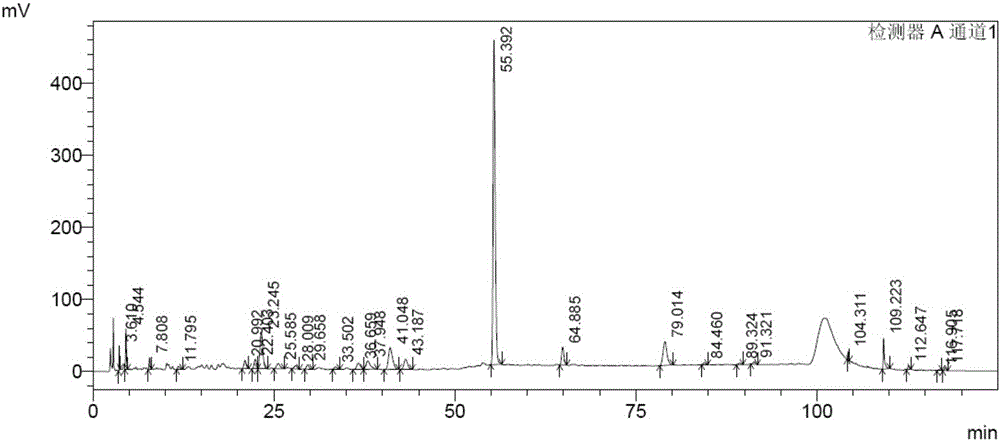
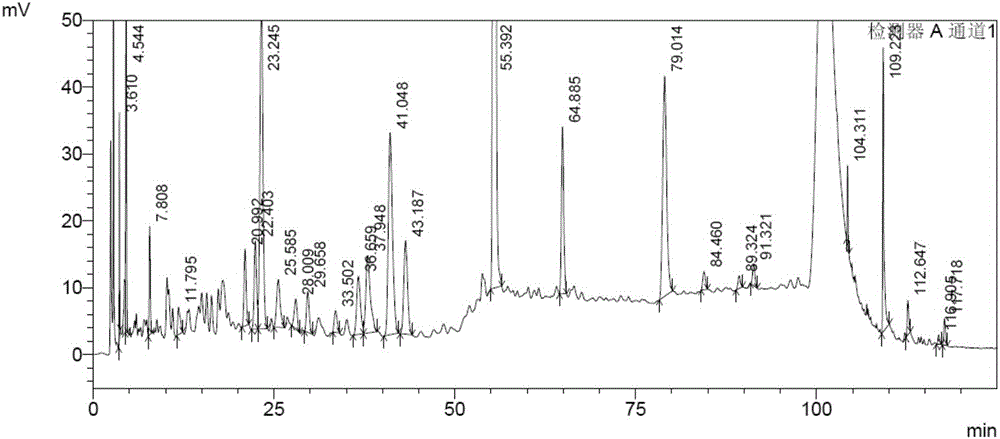
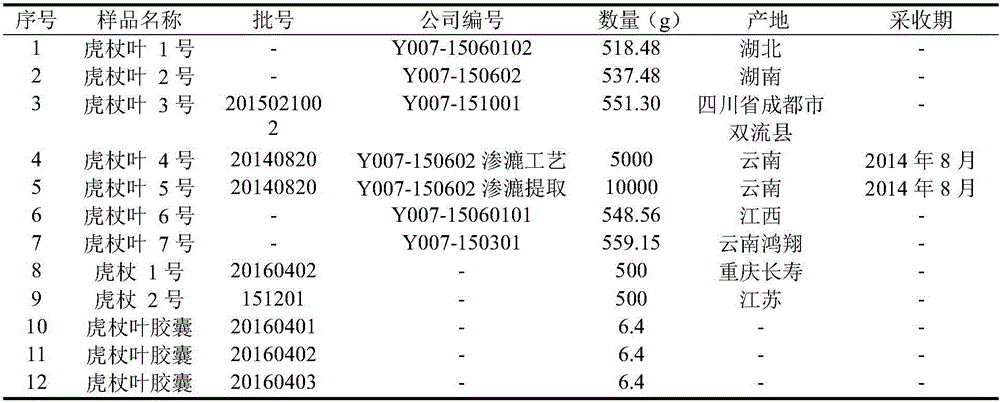
Bushy knotweed leaf fingerprint HPLC method and its use in bushy knotweed leaf capsule quality control
ActiveCN106018629AHigh separation of chromatographic peaksSolving Quality Control IssuesComponent separationGoniothalamusHplc method
The invention provides a bushy knotweed leaf fingerprint HPLC method. The method comprises preparation of a sample to be detected and HPLC detection of the sample. The method can simultaneously detect 12 index components such as gallic acid, chlorogenic acid, ferulic acid, polydatin, rutin, isoquercitrin, quercitrin, resveratrol, quercetin, rheum emodin, chrysophanol and physcion in bushy knotweed leaf raw drugs and their products. The bushy knotweed leaf fingerprint HPLC method has a chromatographic peak resolution, realizes retention time stabilization of different samples and solves the bushy knotweed leaf capsule quality control problems.
Owner:云南海沣药业有限公司
Method for simultaneously detecting multiple sugars and sugar alcohols in dairy products
InactiveCN105675758AReduce churnImprove toleranceComponent separationCorrelation coefficientIsocratic elution
The invention relates to a method for simultaneously detecting multiple sugars and sugar alcohols in dairy products. The method is an HPLC-RID (high performance liquid chromatography-refractive index detection) method and is characterized in that the chromatographic condition of HPLC is as follows: an amido bond column is adopted as a chromatographic column, a single mobile phase of acetone, water and trithylamine is adopted, and isocratic elution is performed; the column temperature is 70 DEG C; the flow velocity is 0.8 ml / min; the sample size is 10 mu L. The detection method is applicable to the dairy products such as milk, cakes, candy, milk powder and the like. Detection can be performed after samples are treated simply. The method is quick, high in recovery rate, wide in linear range, good in correlation coefficient and low in detection limit and fills up the blank of simultaneous detection of the sugars and the sugar alcohols in the dairy products.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for extracting and measuring sulfonamide antibiotics in plant
ActiveCN108760935ANot easy to decomposeTo achieve the effect of cell destructionComponent separationCentrifugationNitrogen
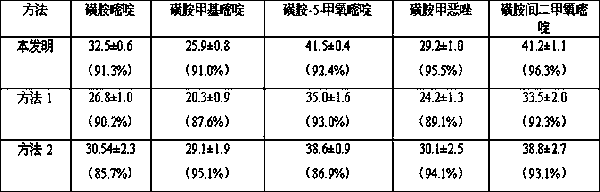
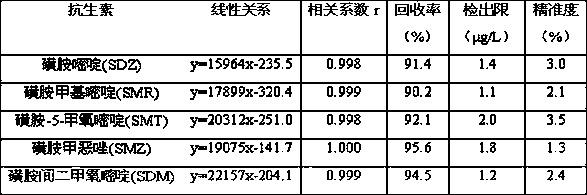
The invention discloses a method for extracting and measuring sulfonamide antibiotics in plant. The method comprises the following specific steps: adding a pretreated plant sample into a methanol / hydrochloric acid mixed extracting solution, sequentially performing ultrasonic, vibration and centrifugation processes, then collecting supernate to be stand-by; continuously adding acetone into a residual, sequentially performing ultrasonic, vibration and vortexprocesses, and collecting supernate; separating the supernate through a solid phase extraction column, ensuring that eluant is methanol, drying the eluant through nitrogen, and dissolving the nitrogen through the methanol; measuring the content of sulfonamide antibiotics in a methanol solving liquid through high performance liquid chromatography. The method overcomes the problems of low recovery rate, complicated test processes and low detection limit in the research of sulfonamide antibiotic residues in the existing plant, and is convenient, accurate and efficient.
Owner:HUAIYIN TEACHERS COLLEGE
Gas chromatography method for simultaneously detecting n-heptane, isooctane, ethyl acetate and isopropanol and use thereof
InactiveCN105806968AConvenient purity testFast purity testComponent separationChemical synthesisGas phase
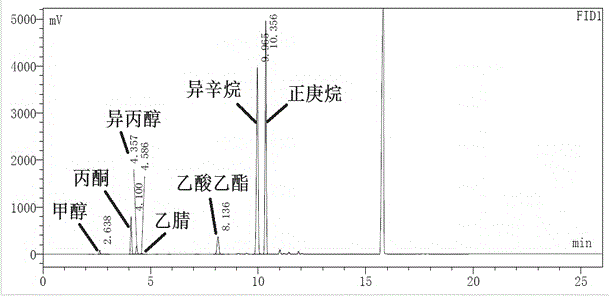
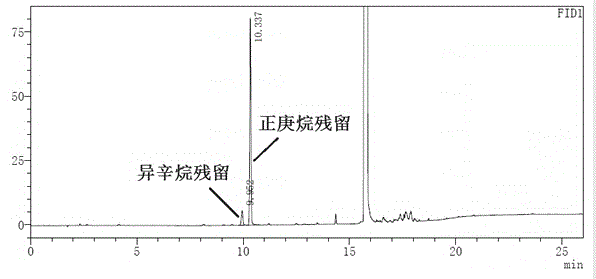
The invention relates to a gas chromatography method for simultaneously detecting n-heptane, isooctane, ethyl acetate and isopropanol and a use thereof. The gas chromatography method can be operated simply, has good peak shapes, high column efficiency, good separation effects, good stability and good reappearance and is suitable for four reagents. The invention discloses the gas chromatography method. The method comprises directly injecting n-heptane, isooctane, ethyl acetate and isopropanol into a gas chromatograph, treating the samples carried by nitrogen through a capillary column chromatographic column with 6% cyanopropylphenyl-94% dimethyl polysiloxane as a fixing solution and carrying out gas chromatography-based qualitative and quantitative analysis on the detected data. The gas chromatography method realizes convenient and fast purity detection of n-heptane, isooctane, ethyl acetate and isopropanol. All the reagents have stable main peak retention time, good peak shapes and good separation effects and satisfy requirements on medicine synthesis or chemical synthesis residual reagent detection and quality control.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Method for simultaneously detecting content of multiple phenolic acids in Noni juice by HPLC (high performance liquid chromatography) wavelength switching technology
ActiveCN104374854AAccurate calculationGood reproducibilityComponent separationFruit juiceGradient elution
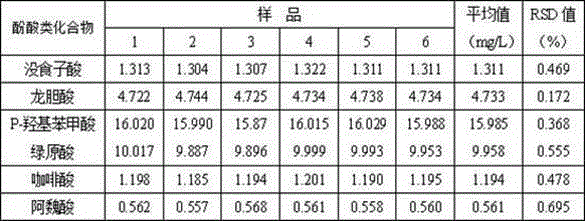
The present invention provides a method for simultaneously detecting content of multiple phenolic acids in Noni juice by HPLC (high performance liquid chromatography) wavelength switching technology, the method includes qualitative and quantitative analysis of the phenolic acids in the Noni juice by the HPLC wavelength switching technology, a C18 column is used, a methanol-acetic acid water solution is used as a mobile phase for gradient elution, a detector is a diode array detector, separation and determination of multiple phenolic acid components can be completed simultaneously by wavelength switching, and a standard substance peak area external standard method is used for quantitation. The method is characterized in that by the use of the wavelength switching technology, interfering substances influence is removed, the content of six phenolic acids in the Noni juice can be detected, determinate components can be well separated within 30min, and the method has high accuracy and good repeatability. The method can effectively separate and accurately determine the multiple phenolic acids in Noni the juice, and has very important significance for the research on the efficacy components in the Noni juice and product quality evaluation.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD +1
Rapid determination method for active component in mulberry tea or mulberry leaf
InactiveCN101806783AEasy to operateStable retention timeComponent separationAdditive ingredientPeak area
A rapid determination method for an active component in the mulberry tea or mulberry leaf relates to a determination method for an active component in the mulberry tea or mulberry leaf. The invention solves the problems that the process is complicated and the ingredients of the mulberry tea or mulberry leaf cannot be truly reflected in the method for preparing the essential oil from the active component in the mulberry tea or mulberry leaf, and the apparatus is expensive and the extraction content is small in the solid phase micro extraction method, which result in the test failure, in the existing pre-treatment method of the mulberry tea or mulberry leaf when testing and detecting the mulberry tea or mulberry leaf by a GC / MS (gas chromatography-mass spectrometer). The method comprises the following steps: 1, placing the mulberry tea or mulberry leaf in a dryer hermetically, aspirating and absorbing by a Tenax-TA absorption tube; 2, connecting a thermal desorption instrument with the GC-MS, placing the absorption tube in the thermal desorption instrument, setting parameters, and performing the determination; 3, calculating the relative content of the active component by a peak-area normalization method. The method is simple and free from property change of the active component, has low-cost apparatus and high detecting success rate up to 100%, and is capable of testing and detecting the mulberry tea or mulberry leaf.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
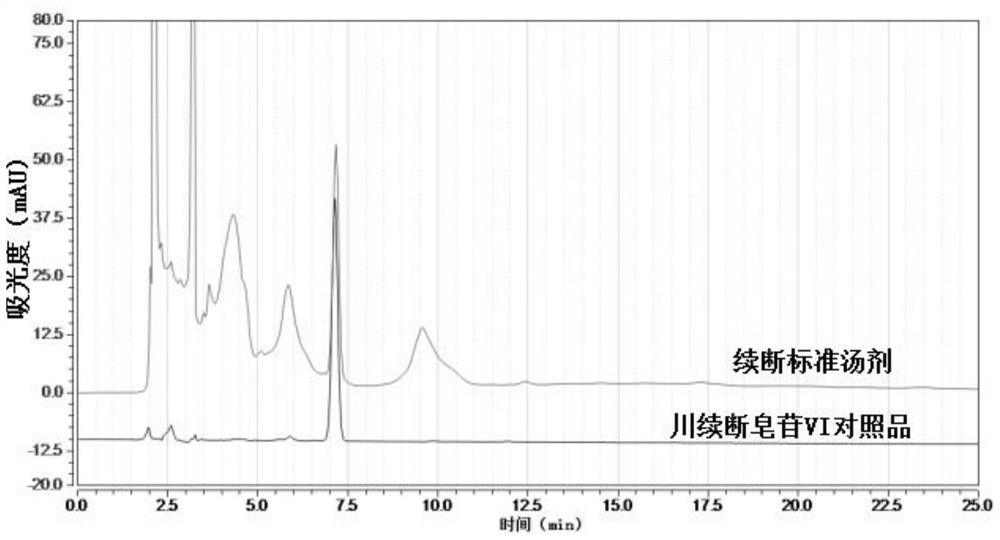
Preparation method and detection method of Himalayan teasel root standard decoction
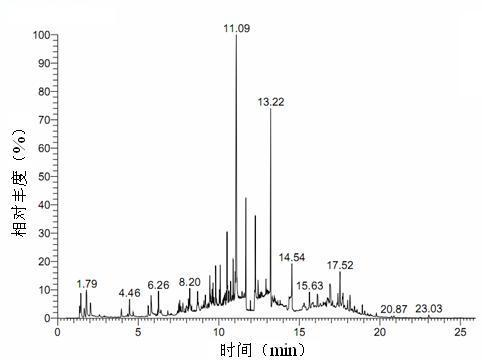
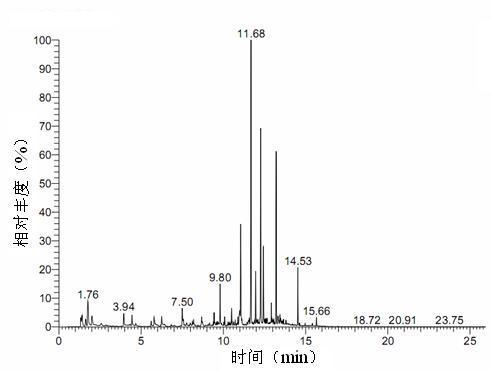
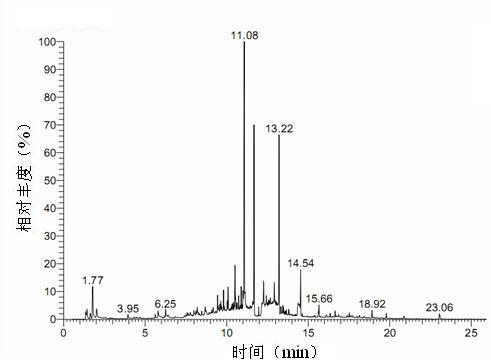
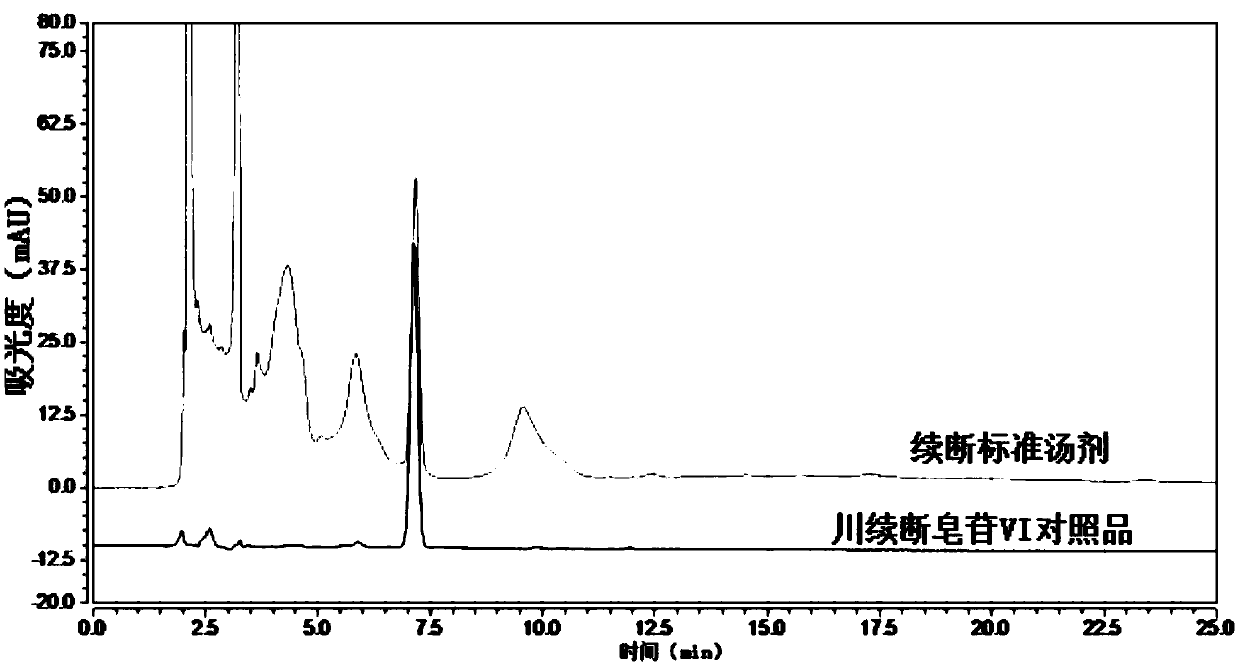
ActiveCN110161135AAchieving quality controlAchieve effectivenessComponent separationFreeze-dryingMoisture absorption
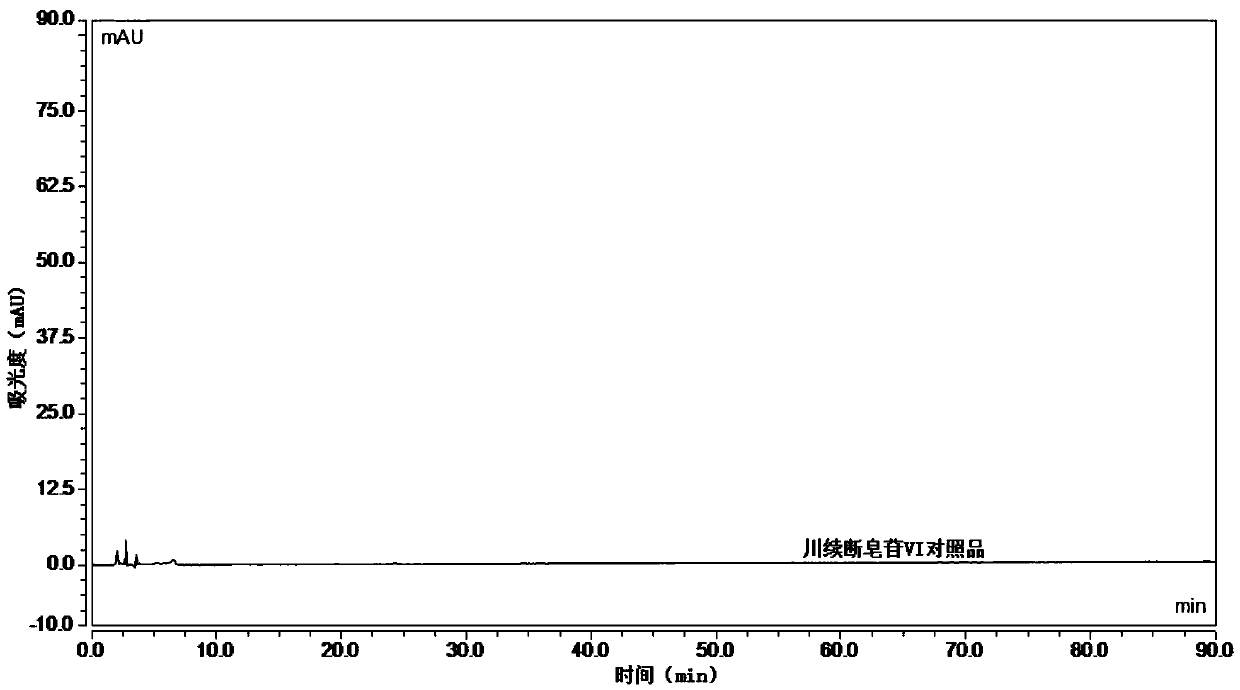
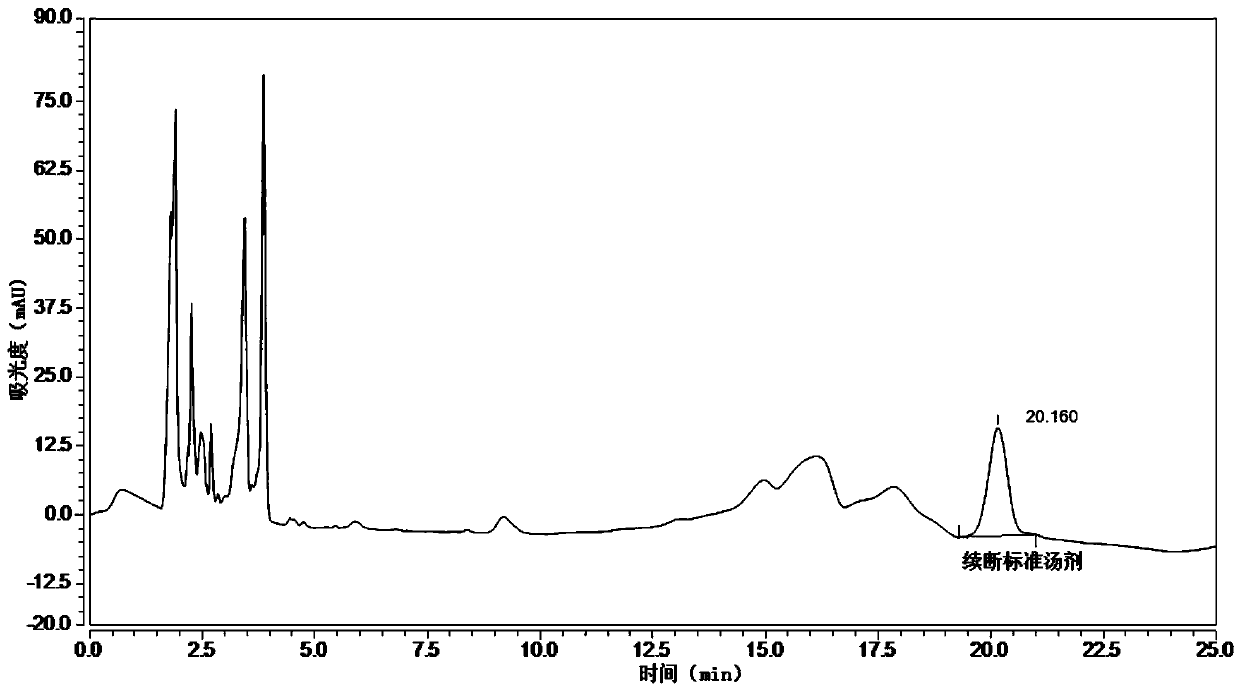
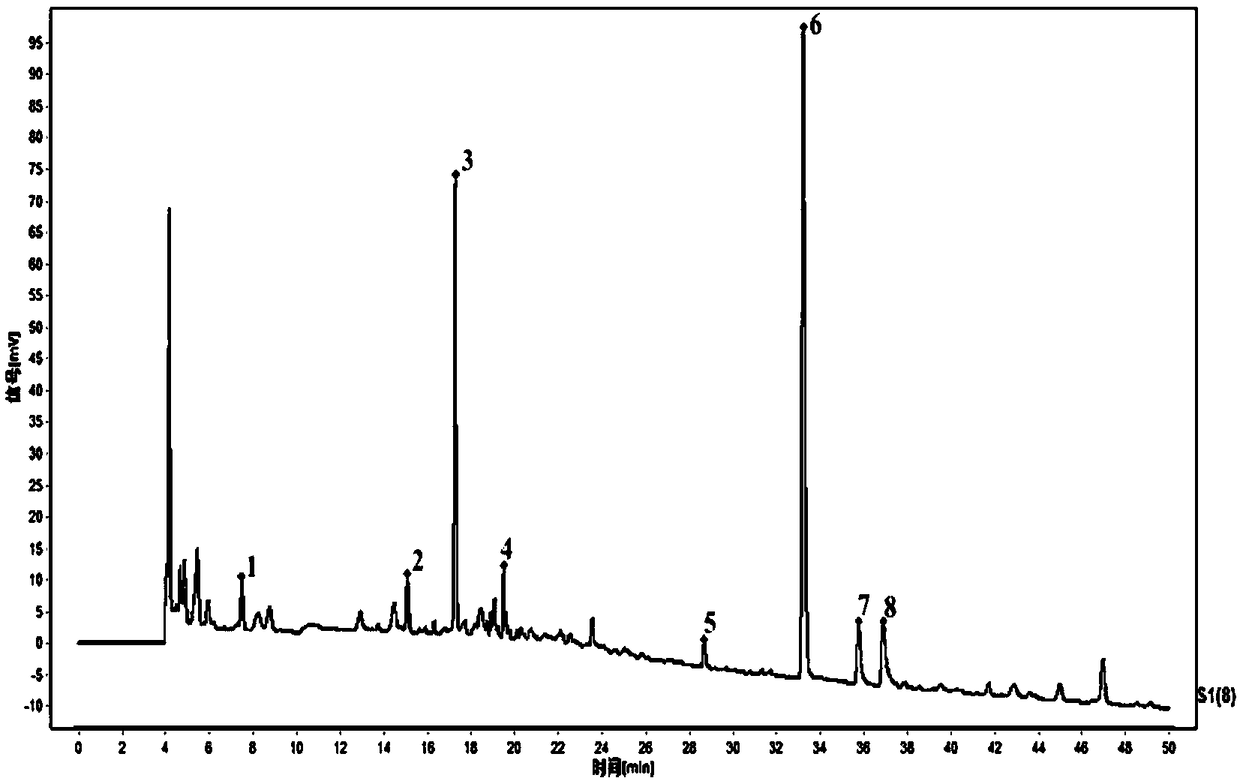
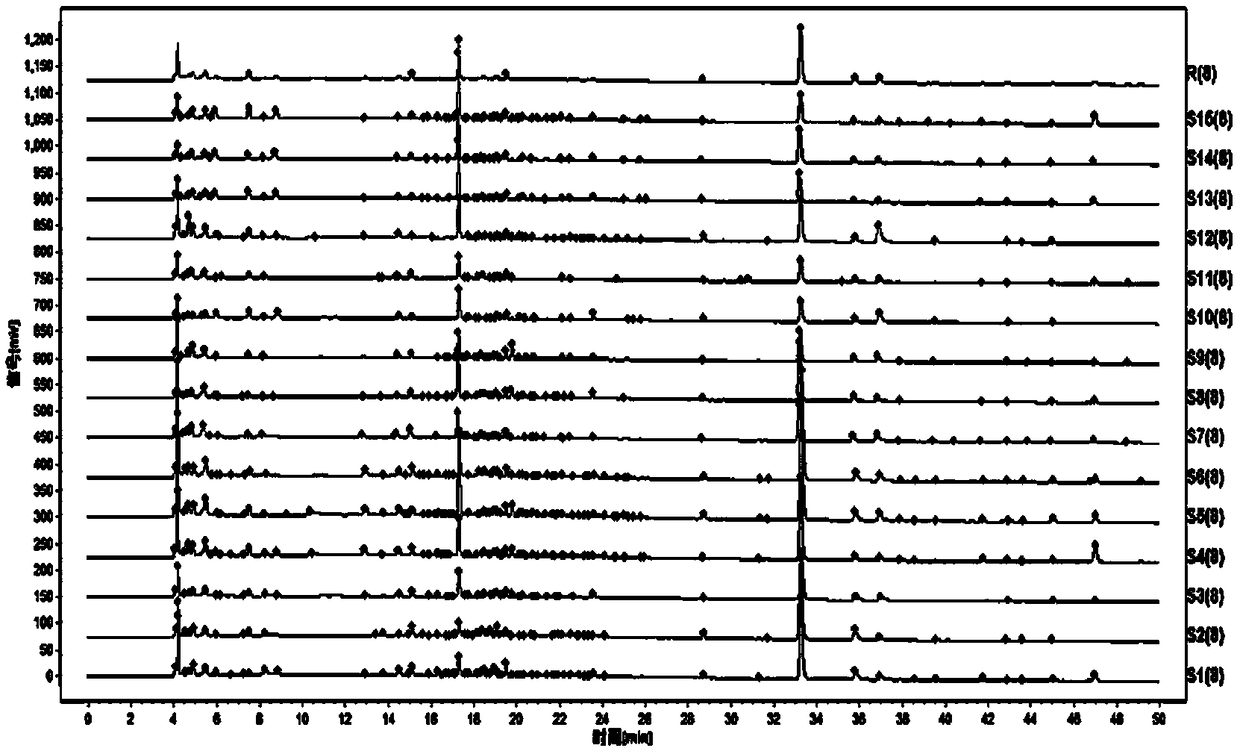
The invention relates to the field of modernization of traditional Chinese medicine, and concretely relates to a preparation method and a detection method of a Himalayan teasel root standard decoction. The invention provides the preparation method of the Himalayan teasel root standard decoction. The preparation method includes the following steps: a Himalayan teasel root medicinal material is taken, decocted with water, and then filtered to obtain a filtrate; and the filtrate is concentrated, dried, and then freeze-dried, wherein the freeze-drying includes the following stages: a. pre-freezingwith pre-freezing temperature being -50 DEG C--45 DEGC; b. primary drying with drying temperature being -20 DEG C-0 DEG C; and c. secondary drying with drying temperature being 5 DEG C-25 DEG C, andthe Himalayan teasel root standard decoction is obtained. The extraction rate of the Himalayan teasel root standard decoction is 32.30-59.98%. Through an accelerated stability test of six months, theHimalayan teasel root standard decoction has stable content and stable moisture content, is moisture absorption-resistant, and does not produce impurity components. The invention provides the detection method of quality, content, transfer rate and characteristic spectrum of Himalayan Teasel Root saponins VI, which has good specificity, repeatability and stability, provides a standard for the quality of traditional Chinese medicine formula granules and achieves the quality control and effective supervision of the traditional Chinese medicine formula granules.
Owner:GUOYAOJITUAN TONGJITANG (GUIZHOU) PHARMA CO LTD +2
Radix aconiti carmichaeli fingerprint spectrum, establishment method of radix aconiti carmichaeli fingerprint spectrum, and radix aconiti carmichaeli quality detection method
The invention relates to the technical field of drug detection, and particularly relates to a radix aconiti carmichaeli fingerprint spectrum, an establishment method of the radix aconiti carmichaeli fingerprint spectrum, and a radix aconiti carmichaeli quality detection method. The establishment method of the radix aconiti carmichaeli fingerprint spectrum mainly comprises the following steps of preparing a reference solution, and preparing a radix aconiti carmichaeli test solution; and measuring the radix aconiti carmichaeli test solution and the reference solution by high performance liquid chromatography. The method provided by an embodiment of the invention is stable, reliable, simple to operate and good in repeatability. The fingerprint spectrum analysis method parameters provided by the invention can meet relatively high requirements in precision, repeatability, durability and specificity, so that the stability and reliability of the method are proved.
Owner:YAAN THREE NINE PHARMA
Fingerprint spectrum detection method and quality control method of capillary artemisia formula granules
ActiveCN106932523AUniform separationEasy to separateComponent separationChlorogenic acidGradient elution
The invention relates to the field of quality inspection of capillary artemisia formula granules, and provides a fingerprint spectrum detection method of the capillary artemisia formula granules. The method includes the steps that chlorogenic acid is obtained for preparing a reference solution, capillary artemisia medicinal materials are obtained for preparing a multiple-node-sample solution, and high performance liquid chromatograph is conducted on the reference solution and the multiple-node-sample solution respectively, and the chromatographic conditions are as follows: an aqueous solution of methanol and acetic acid is used as a mobile phase, the reference solution and the multiple-node-sample solution are allowed to pass a chromatographic column respectively and subjected to gradient elution, the methanol volume is controlled in the gradient elution process, and the percentage, in the total amount of the le phase, of methanol volume is increased from 4-6% to 40-50% and then is reduced to 4-6%. The fingerprint spectrums of a product in all stages of the capillary artemisia formula granules can be established, and the quality of the capillary artemisia formula granules can be well controlled. Moreover, the invention provides a quality control method, including the capillary artemisia formula granule fingerprint spectrum detection method, of the capillary artemisia formula granules.
Owner:HARBIN ZHENBAO PHARMA +1
Analysis method for determining releasing rate of quetiapine fumarate sustained release tablet
InactiveCN110133134AStable retention timeGood peak shapeComponent separationAnalysis methodAnalytical chemistry
The invention discloses an analysis method for determining releasing rate of a quetiapine fumarate sustained release tablet. The method is about determining releasing rate of the quetiapine fumarate sustained release tablet, dissolution medium in the releasing rate method is composed of water, buffer salt and surfactant, and a liquid chromatography is used for analyzing a sample for test. The dissolution medium in the determining method can improve releasing quantity of the quetiapine fumarate sustained release tablet in a releasing rate experiment, build a proper chromatographic condition andachieve an aim of analysis.
Owner:NOVAST LABORATORIES (CHINA) LTD
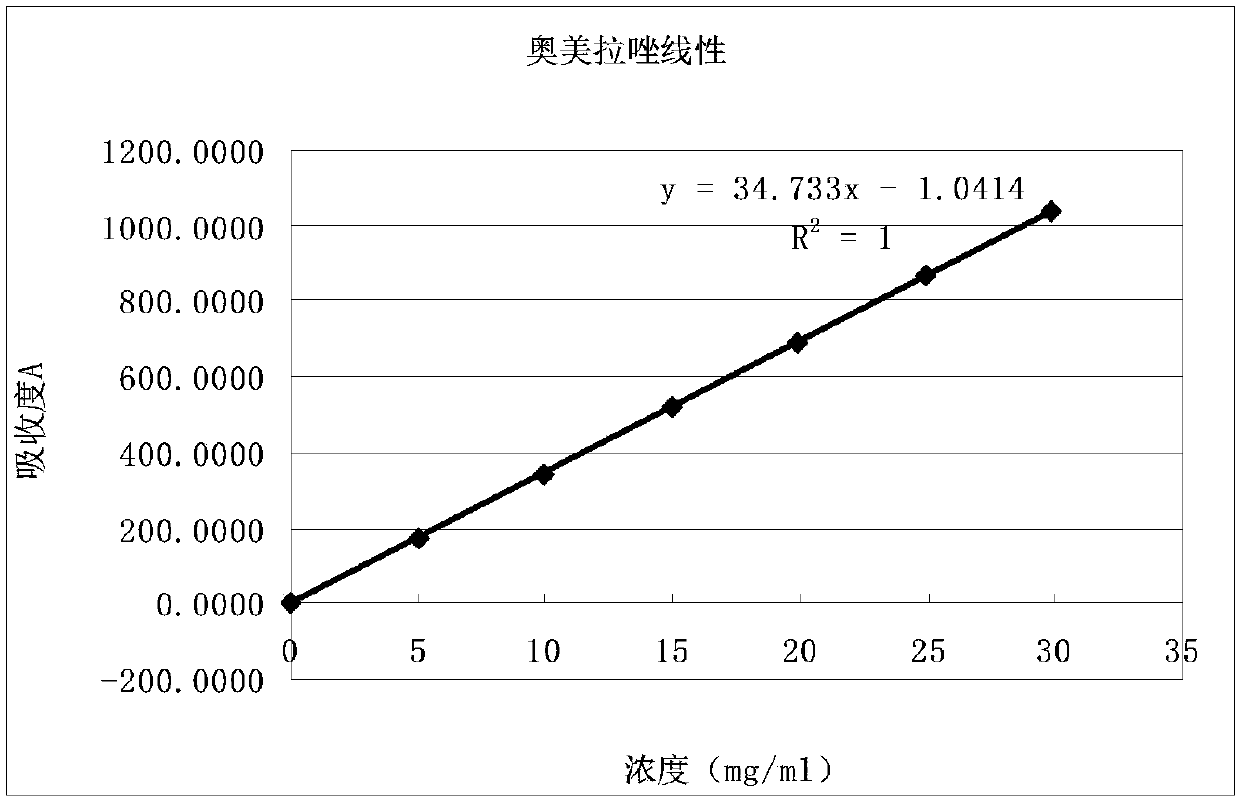
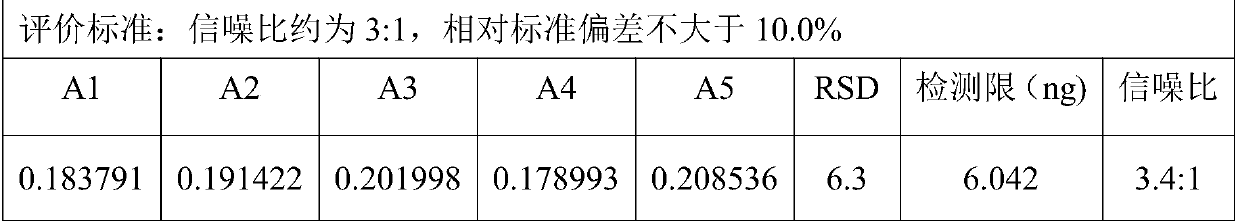
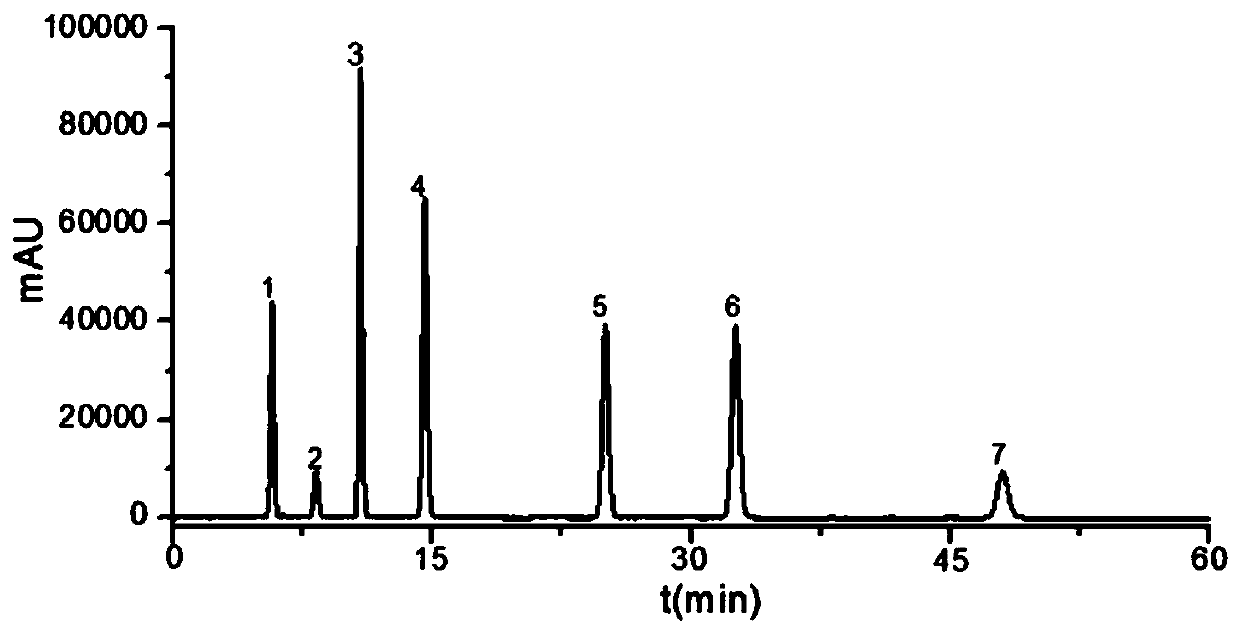
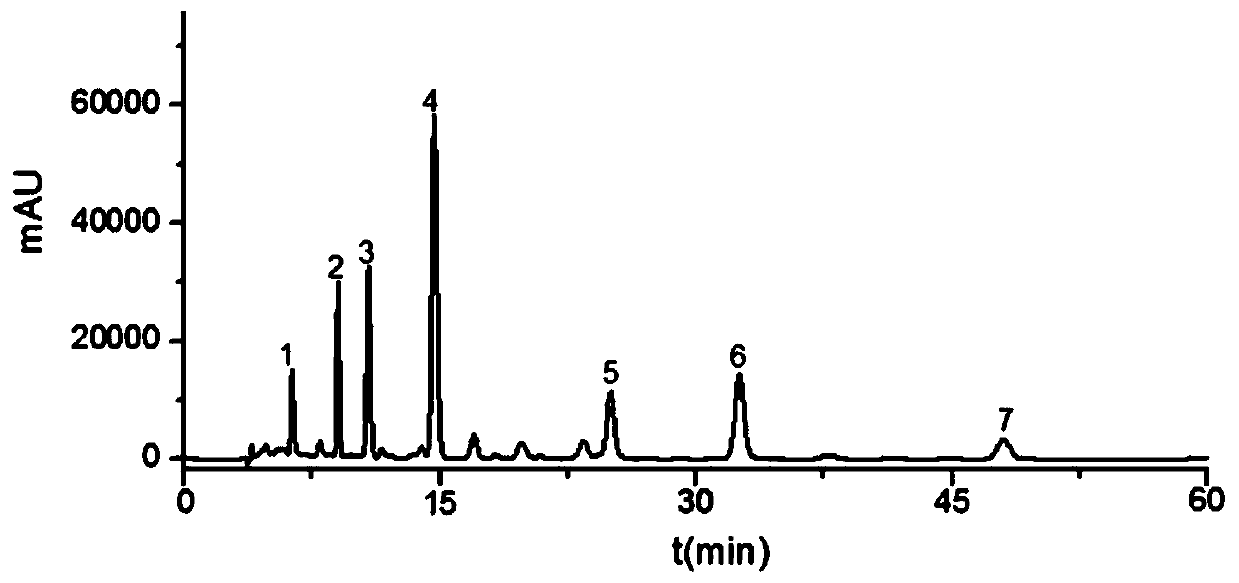
Determination method of main drug content in omeprazole solid preparation
The invention provides a determination method of a main drug content in an omeprazole solid preparation. A mixed solvent of sodium heptanesulfonate and acetonitrile is used as a mobile phase, methyl alcohol is used as a diluent, and high performance liquid chromatography (HPLC) is adopted for detecting. The determination method provided by the invention is subjected to methodology validation, andthe determination method of the main drug content in the omeprazole solid preparation provided by the invention has a great advantage on sample detection compared with a determination method of an existing detection technology on the content in the omeprazole solid preparation, so that the retention time of omeprazole can be stable, the separation degree is favorable, and the number of theoreticalplates can reach to 10000 or more; the solubleness of the omeprazole by methyl alcohol is favorable, and the problem of caking is solved; meanwhile, the error of manual operation is reduced, and thedetection accuracy is improved.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
High performance liquid chromatography (HPLC) wavelength switching technology-based method for simultaneously measuring content of various constituents in san huang tablet
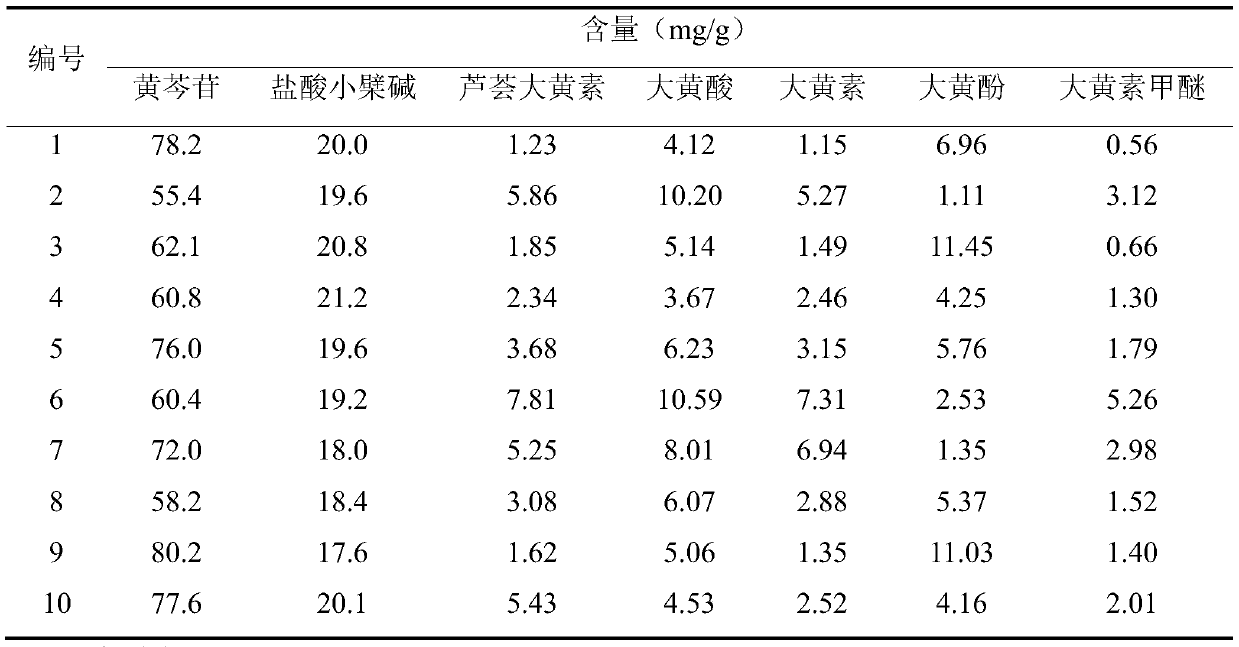
InactiveCN110646537ASimple and fast operationHigh detection sensitivityComponent separationPhosphoric acidBerberine hydrochloride
The invention discloses a high performance liquid chromatography (HPLC) wavelength switching technology-based method for simultaneously measuring content of various constituents in san huang tablet, and relates to the technical field of drum detection method. The HPLC wavelength switching technology-based method comprises the following steps of simultaneously performing qualitative and quantitative analysis on seven types of constituents comprising baicalin, berberine hydrochloride, aloe-emodin, rhein, emodin and physcion; preparing a mixed contrast solution; preparing a sample solution; performing HPLC separation and detection; and performing qualitative and quantitative analysis on a sample. An HPLC wavelength switching technology is employed, detection is performed when baicalin wavelength is switched at 280 nanometers, detection is performed when berberine hydrochloride wavelength is switched at 264 nanometers, detection is performed when wavelengths of the aloe-emodin, the rhein,the emodin and the physcion are switched at 254 nanometers, an elution condition is in a way that mobility-phase A acetonitrile and mobility-phase B phosphate solution and is as follows: 15% of A and85% are contained of B when 0-10 minutes are passed, 25% of A and 75% of B are contained when 20 minutes are passed, 40% of A and 60% of B are contained when 30 minutes as passed, 55% of A and 45% B are contained when 42 minutes are passed, and 80% of A and 20% of B are contained when 50-60 minutes are passed. The HPLC wavelength switching technology-based method is simple to operate and is high in detection sensitivity and more accurate in detection result.
Owner:陕西起源农业科技有限责任公司
Ophiopogon japonicus fingerprint, construction method thereof and ophiopogon japonicus quality detection method
ActiveCN109541098AStable retention timeAvoid inseparable problemsComponent separationChemical compositionRetention time
The invention relates to the technical field of pharmaceutical quality detection, in particular to an ophiopogon japonicus fingerprint, a construction method thereof and an ophiopogon japonicus quality detection method. The construction method of the ophiopogon japonicus fingerprint mainly comprises a step of determining a reference solution and a test solution prepared from the ophiopogon japonicus with high performance liquid chromatography, wherein the chromatographic conditions are as follows: octadecyl silane bonded silica gel is utilized as a chromatography column of a filling agent; a mobile phase A is acetonitrile; a mobile phase B is a trifluoroacetic acid aqueous solution; and gradient elution is performed on the reference solution and the test solution respectively. The fingerprint provided in this embodiment can systematically and comprehensively reflect various chemical components in ophiopogon japonicus, the common peak has stable relative retention time, and the ophiopogon japonicus fingerprint has good stability. According to the ophiopogon japonicus fingerprint, the construction method thereof and the ophiopogon japonicus quality detection method, the analysis process of the ophiopogon japonicus and its preparations are simplified, the relevance of the ophiopogon japonicus is accurately evaluated, and the quality of the ophiopogon japonicus and preparations canbe controlled.
Owner:YAAN THREE NINE PHARMA
Analysis method for content of 5-chloro-2-methoxycarbonyl-1-indanone ester
The invention belongs to the technical field of chemical analysis and relates to ananalysis method for the content of 5-chloro-2-methoxycarbonyl-1-indanone ester. According to the analysis method, high performance liquid chromatography is adopted to detect the content of the 5-chloro-2-methoxycarbonyl-1-indanone ester; an SBC8 reversed phase chromatographic column is adopted as a chromatographic column; the temperature of the chromatographic column is 30 DEG C to 50 DEG C; a mobile phase is a mixed system of acetonitrile and ammonium carboxylate; the detection wavelength is 230 nm and 270 nm;impurities, a main peak and a solvent peak can be completely separated; the chromatographic peak shape is good; the retention time is stable; the integral calculation result is accurate; the repeatability is good; the reliability of the obtained result is high; and the method is particularly suitable for quality control of pesticide raw drug intermediate products.
Owner:JINGBO AGROCHEM TECH CO LTD
LC-MS/MS detection method for gallotannin in plants
PendingCN114740100AHigh sensitivityExclude a large number of interfering ionsComponent separationTandem mass spectrometryMass spectrum analysis
The invention belongs to the technical field of compound detection, and provides an LC-MS / MS (liquid chromatography-mass spectrometry / mass spectrometry) detection method for gallotannin in plants, which comprises the following steps: mixing a plant gallotannin sample and a methanol solution, and sequentially extracting and centrifuging to obtain a sample to be detected; carrying out liquid chromatography separation and tandem mass spectrometry detection on a to-be-detected sample, and establishing a mass spectrometry database of gall tannin compounds; mRM quantitative ion pairs are obtained through screening according to MS / MS information, and optimized MRM mass spectrum conditions are obtained through a plant gallnut tannin sample; ion pairs and retention time of gallotannin compounds are integrated, and MRM mass spectrum conditions are optimized, so that qualitative and quantitative detection of gallotannin in plants is realized. According to the detection method disclosed by the invention, the detection process of the gallotannin compound does not depend on a chemical standard substance, the detection method has the advantages of comprehensive detection, high sensitivity, good repeatability, high accuracy, simplicity in operation and the like, and qualitative and quantitative analysis is realized at the same time.
Owner:NORTHWEST A & F UNIV
A HPLC detection method of gibberellin content in sugarcane leaves
InactiveCN106442792BImprove purityGood effect of removing impuritiesComponent separationHplc methodBiochemical mechanism
The invention discloses an HPLC determination method for the gibberellin content in sugarcane leaves. The HPLC detection method comprises the following steps: (S1) adding liquid nitrogen into to-be-detected sugarcane leaves, grinding, carrying out methanol extraction and centrifugation, extracting supernate, extracting residues with methanol, extracting supernate after the centrifugation, combining the types of supernate, carrying out reduced pressure vaporization, carrying out petroleum ether extraction and decoloration, carrying out reduced pressure steaming on a low-layer water phase, adding a flow phase to dissolve, carrying out volume fixation, and filtering to obtain liquid as a sample solution for later use; and (S2) determining the gibberellin content in the sample solution by virtue of an HPLC. By virtue of the HPLC determination method, the gibberellin content in the sugarcane leaves can be determined, and the scientific basis is provided for the research of a biochemical mechanism of the gibberellin in the mediation of the stress response of pathogenic bacteria of the top rot of the sugarcanes.
Owner:SUGARCANE RES INST GUANGXI ACADEMY OF AGRI SCI
A HPLC detection method of salicylic acid content in sugarcane leaves
InactiveCN106526037BImprove purityActive ingredient completeComponent separationBiochemical mechanismHplc method
Owner:SUGARCANE RES INST GUANGXI ACADEMY OF AGRI SCI
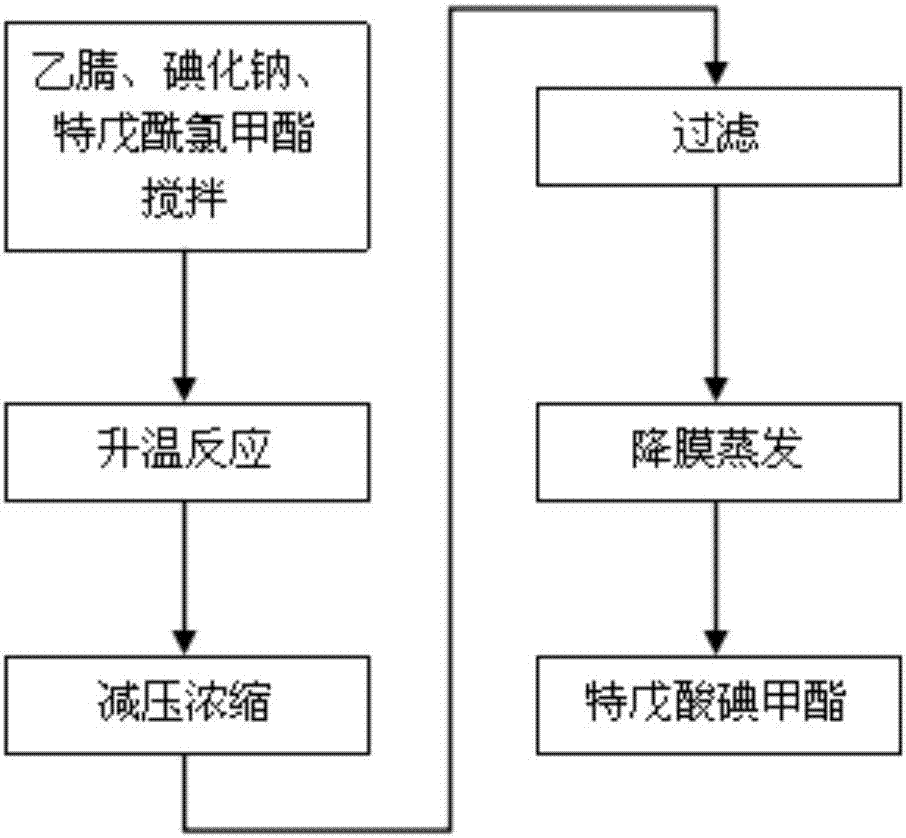
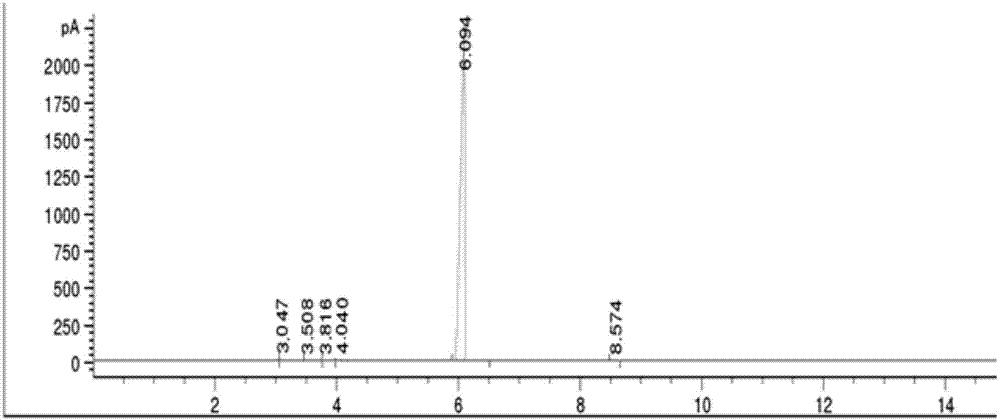
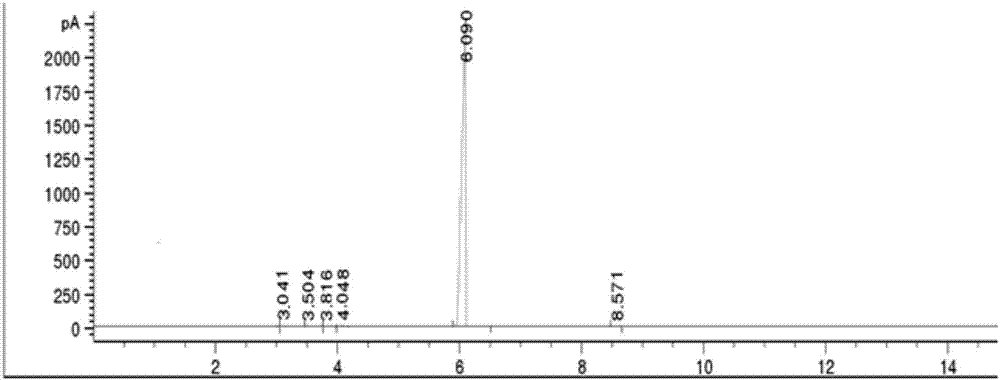
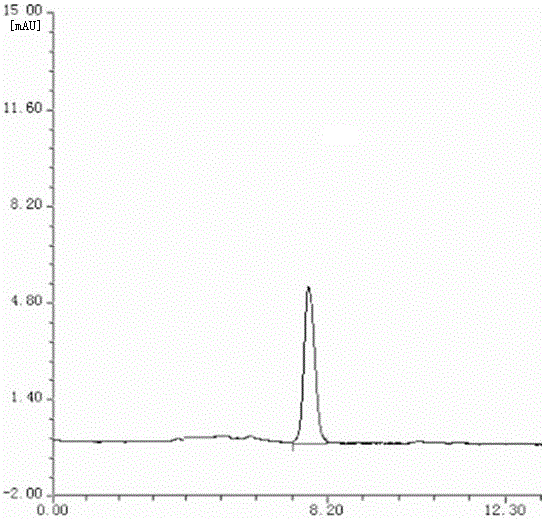
Gas chromatography detection method and preparation method of iodometyl pivalate
ActiveCN107422056AQuality improvementQuick responseOrganic compound preparationComponent separationGas phaseChromatographic column
The invention belongs to the technical field of pharmacochemistry, and particularly relates to a gas chromatography detection method and a preparation method of iodometyl pivalate. The detection method comprises the following steps of (1) storing a sample to be tested under the light avoiding and drying conditions; (2) setting the work conditions of a gas chromatograph, wherein a chromatographic column selects a KB-5 capillary chromatographic column; the column box temperature is 110 to 220 DEG C; the sampling opening temperature is 220 DEG C; the temperature of a detector is 240 DEG C; the flow rate of carrier gas is 0.3 to 0.7 ml / min; the split ratio is 80 to 1; the sampling quantity is 0.1mu L. By the preparation method of samples for detection, a treated iodometyl pivalate solution passes through a falling-film evaporator to obtain high-quality iodometyl pivalate. The analysis speed of the detection method is high; the detection sensitivity is high; the analysis result is accurate; no three wastes (waste water, waste gas and waste residue) are generated in the preparation process; the product purity is high.
Owner:SHANDONG YUXIN PHARMA CO LTD
HPLC determination method for gibberellin content in sugarcane leaves
InactiveCN106442792AImprove purityGood effect of removing impuritiesComponent separationBiochemical mechanismCentrifugation
The invention discloses an HPLC determination method for the gibberellin content in sugarcane leaves. The HPLC detection method comprises the following steps: (S1) adding liquid nitrogen into to-be-detected sugarcane leaves, grinding, carrying out methanol extraction and centrifugation, extracting supernate, extracting residues with methanol, extracting supernate after the centrifugation, combining the types of supernate, carrying out reduced pressure vaporization, carrying out petroleum ether extraction and decoloration, carrying out reduced pressure steaming on a low-layer water phase, adding a flow phase to dissolve, carrying out volume fixation, and filtering to obtain liquid as a sample solution for later use; and (S2) determining the gibberellin content in the sample solution by virtue of an HPLC. By virtue of the HPLC determination method, the gibberellin content in the sugarcane leaves can be determined, and the scientific basis is provided for the research of a biochemical mechanism of the gibberellin in the mediation of the stress response of pathogenic bacteria of the top rot of the sugarcanes.
Owner:SUGARCANE RES INST GUANGXI ACADEMY OF AGRI SCI
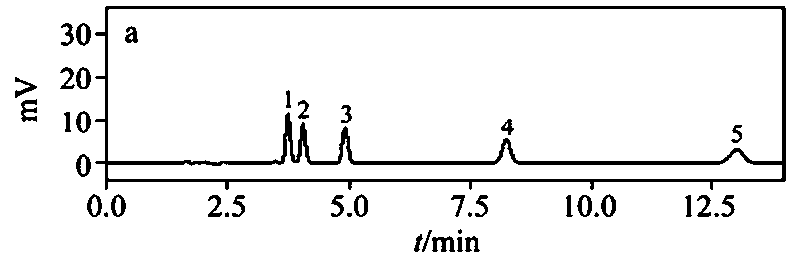
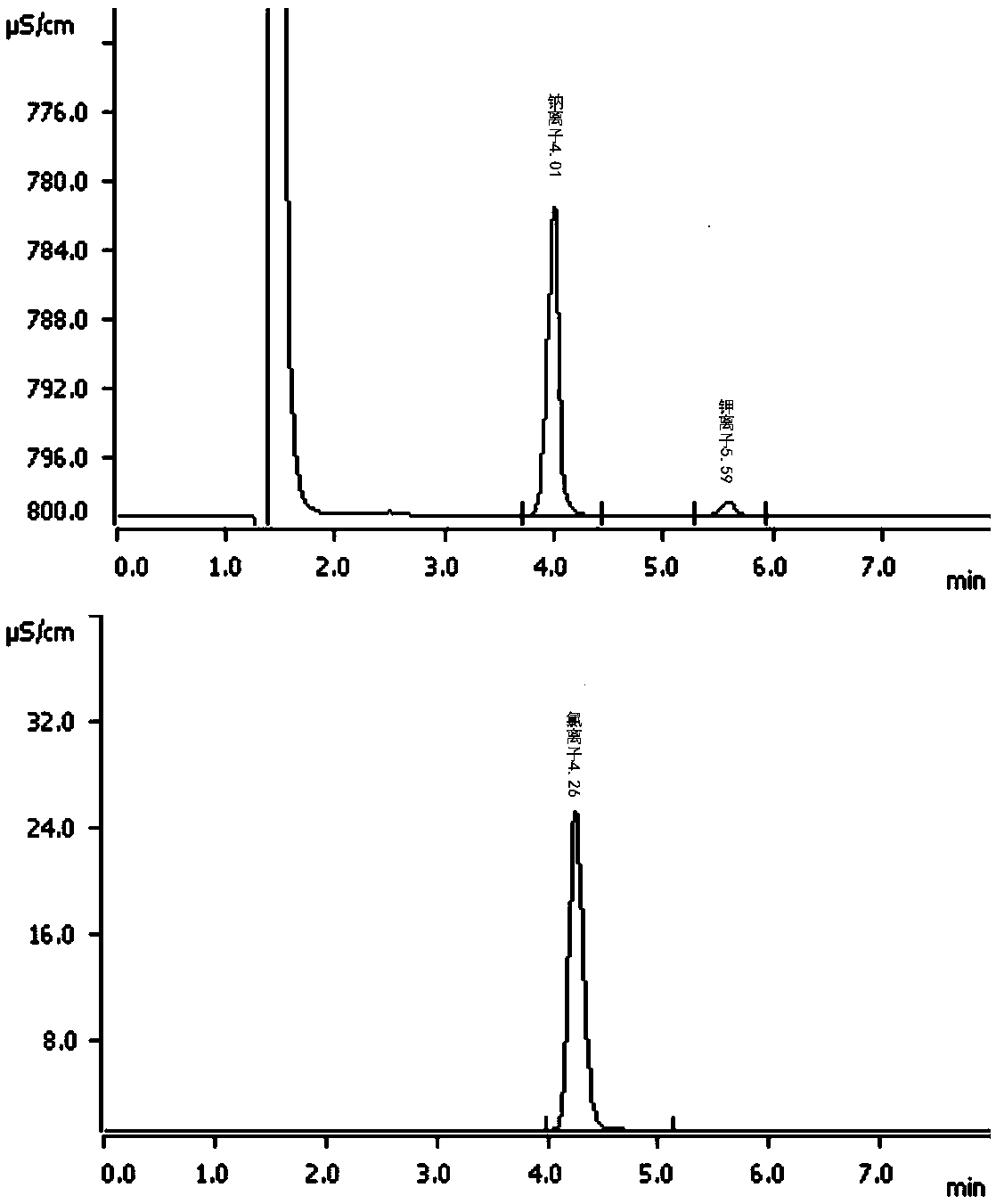
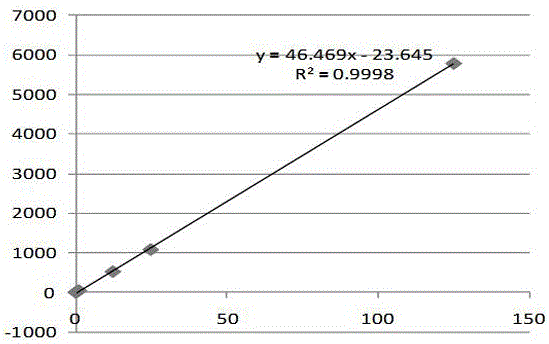
Determination method of anion and cation content in polyethylene glycol electrolyte preparation
ActiveCN106568886BImprove detection efficiencySimple methodComponent separationSodium bicarbonateOrganic solvent
The invention belongs to the field of medicine, and specifically provides a method of using HPLC (High Performance Liquid Chromatography) to measuring the contents of cations and anions in a polyethylene glycol electrolyte preparation. The chromatographic conditions are as follows: the cation eluent is a nitric acid water solution (2.3-2.7 M) containing a water soluble organic solvent accounting for 5 to 20 vol.% of the eluent; the flowing speed is 0.8 to 1.2 mL / min; the anion eluent is a water solution of sodium carbonate (3.2-3.8 mM) and sodium bicarbonate (0.9-1.1 mM), the flowing speed is 0.6 to 0.8 mL / min, and the detector is an electrical conductivity detector. The measuring method has the advantages of strong specificity, simple and quick operation, capability for prolonging the service life of chromatographic column, and high sensitivity.
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD +1
HPLC detection method of content of salicylic acid in sugarcane leaves
InactiveCN106526037AImprove purityActive ingredient completeComponent separationBiochemical mechanismEvaporation
The invention discloses an HPLC detection method of the content of salicylic acid in sugarcane leaves. The method comprises the following steps: S1, adding the sugarcane leaves to be detected into liquid nitrogen, grinding the leaves, carrying out methanol extraction, centrifuging the obtained extract, taking obtained supernatant, carrying out methanol extraction on obtained residues, centrifuging the residue extract, taking obtained supernatant, mixing the supernatants, carrying out reduced pressure evaporation, carrying out extraction decolorizing, carrying out reduced pressure evaporation on obtained lower layer water phase, adding a mobile phase to dissolve the evaporated water phase, allowing the volume of the obtained solution to reach a constant value, and filtering the solution to obtain a sample solution for later use; and 2, detecting the content of salicylic acid in the sample solution through adopting HPLC. The method allows the content of salicylic acid in sugarcane leaves to be accurately detected, and provides a scientific and powerful detection technology for the top rot resistance difference research of sugarcane varieties and the stress response biochemical mechanism of salicylic acid mediated sugarcane top rot pathogens.
Owner:SUGARCANE RES INST GUANGXI ACADEMY OF AGRI SCI
A kind of high performance liquid phase analysis method of diethylphosphonoacetic acid
The invention discloses a high-efficiency liquid phase analysis method of diethylphosphonoacetic acid. The high-efficiency liquid phase analysis method is characterized by specifically comprising the following steps of using 10% acetonitrile to dissolve and dilute the diethylphosphonoacetic acid into a solution which contains 0.5mg to 50mg of diethylphosphonoacetic acid per ml, wherein the solution is used as a test sample solution, filling a high performance liquid chromatograph with the solution, and setting the sampling volume to 1 to 100mu l; by utilizing an ultraviolet detector with detection wavelength of 200 to 230nm, using an acetonitrile-phosphate solution as a flowing phase with the flow rate of 0.5 to 3.0ml / min and using a fixed-phase octadecyl silane chromatographic column with column temperature of 30 DEG C, measuring the chromatographic purity of the diethylphosphonoacetic acid; according to a peak area, calculating the purity and content of a sample. The high-efficiency liquid phase analysis method for measuring has the advantages that the method is simple, the precision is high, the stability is good, and the reoccurrence is good.
Owner:JIANGSU WANBANG BIOPHARMLS
A method for simultaneously detecting multiple sugars and sugar alcohols in food
InactiveCN105717207BImprove applicabilityStable baselineComponent separationAlcohol sugarsColumn temperature
The invention relates to a method for simultaneously detecting multiple sugars and sugar alcohols in food. An HPLC-differential refraction detection method is adopted as the method; the method is characterized by comprising the HPLC chromatographic conditions that an amide-bonded column is adopted as a chromatographic column, single mobile phases of acetone, water and triethylamine are adopted as mobile phases, isocratic elution is performed, the column temperature is 70 DEG C, the flowing velocity is 0.8 ml / min, and the sample injection amount is 10 milliliters. The detection method can be suitable for the food such as honey, wine and baijiu; detection can be performed only by simply processing a sample, the method is rapid, high in recovery rate, wide in linear range, good in correlation coefficient and low in detection limit, and the blank of simultaneous detection on the sugars and sugar alcohols in the food in China is filled up.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
High-efficiency liquid phase analysis method of diethylphosphonoacetic acid
The invention discloses a high-efficiency liquid phase analysis method of diethylphosphonoacetic acid. The high-efficiency liquid phase analysis method is characterized by specifically comprising the following steps of using 10% acetonitrile to dissolve and dilute the diethylphosphonoacetic acid into a solution which contains 0.5mg to 50mg of diethylphosphonoacetic acid per ml, wherein the solution is used as a test sample solution, filling a high performance liquid chromatograph with the solution, and setting the sampling volume to 1 to 100mu l; by utilizing an ultraviolet detector with detection wavelength of 200 to 230nm, using an acetonitrile-phosphate solution as a flowing phase with the flow rate of 0.5 to 3.0ml / min and using a fixed-phase octadecyl silane chromatographic column with column temperature of 30 DEG C, measuring the chromatographic purity of the diethylphosphonoacetic acid; according to a peak area, calculating the purity and content of a sample. The high-efficiency liquid phase analysis method for measuring has the advantages that the method is simple, the precision is high, the stability is good, and the reoccurrence is good.
Owner:JIANGSU WANBANG BIOPHARMLS
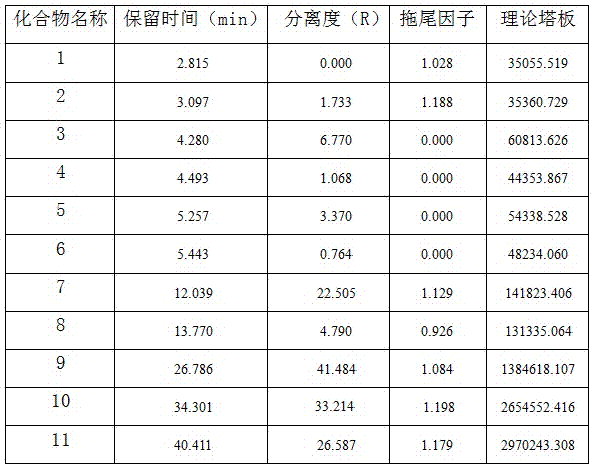
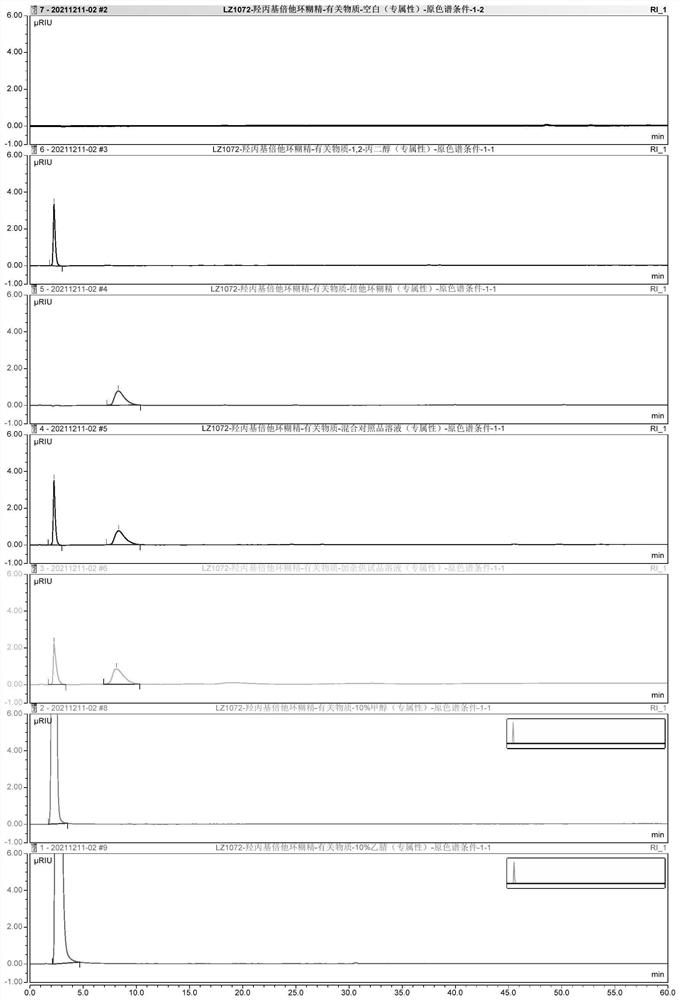
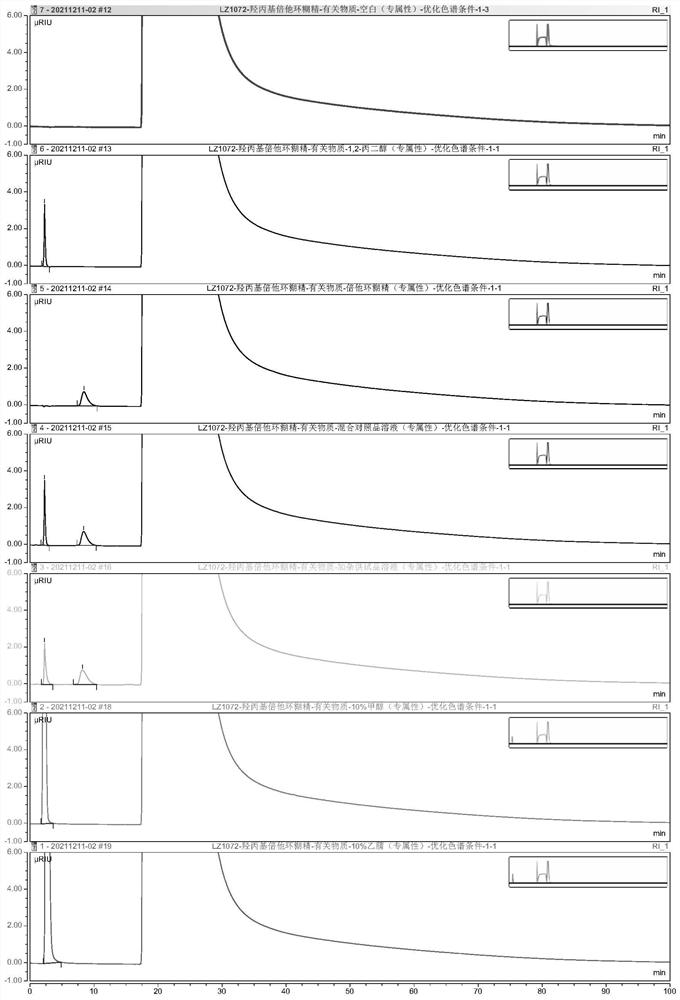
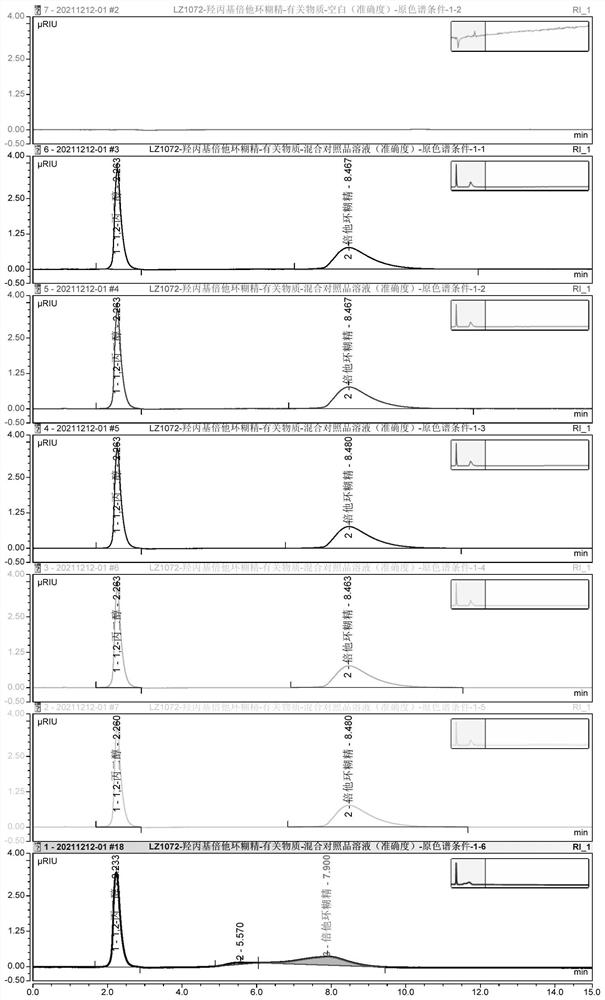
Detection method and application of related substances in hydroxypropyl-beta-cyclodextrin
PendingCN114354795AIncreased durabilityShort retention timeComponent separationFluid phasePhysical chemistry
The invention provides a detection method and application of related substances in hydroxypropyl beta-cyclodextrin, the related substances comprise 1, 2-propylene glycol and beta-cyclodextrin, liquid chromatography is adopted for detection, and the conditions are as follows: a phenyl bonded silica gel chromatographic column; a mobile phase A is water; the mobile phase B is methanol or acetonitrile; an elution procedure: eluting for at least 15 minutes by using the mobile phase A; switching the mobile phase, so that the volume fraction of the mobile phase A in the mobile phase A and the mobile phase B is reduced from 100% to 0-60%; maintaining elution of the switched mobile phase for a period of time; switching the mobile phase, so that the volume fraction of the mobile phase A in the mobile phase A and the mobile phase B is increased from 0-60% to 100%; and maintaining the mobile phase A to elute for a period of time. The method is good in specificity, applicability, accuracy, precision and durability and good in repeatability, and solves the problem that the related substances in the hydroxypropyl-beta-cyclodextrin are difficult to accurately detect under a continuous sample injection condition in the existing method.
Owner:LI MIN PHARM FAB OF LIVZON PHARM GRP
High performance liquid chromatography method for determining related substances in bifonazole bulk drug
PendingCN114252536AHigh separation selectivityGood repeatabilityComponent separationSignal responseFluid phase
The invention provides a high performance liquid chromatography method for determining related substances in a bifonazole bulk drug. The high performance liquid chromatography method for determining the related substances in the bifonazole bulk drug comprises the following steps: 1) dissolving a to-be-determined sample of the bifonazole bulk drug in a mobile phase, and filtering to obtain a to-be-determined solution; and 2) measuring the standard solution and the solution to be measured by using a high performance liquid chromatography by using a mixed solution of a buffer solution and a solvent as a mobile phase to obtain a standard curve, and analyzing the contents of the bifonazole and the related substances in the solution to be measured according to the standard curve. The high performance liquid chromatography method disclosed by the invention is good in separation selectivity, symmetrical in peak shape, linear in quantitative curve, good in repeatability, and stable in retention time, separation degree and signal response value.
Owner:纳谱分析技术(苏州)有限公司
Preparation method and detection method of Dipsacus standard decoction
ActiveCN110161135BAchieve effectivenessStable feature mapComponent separationBiotechnologyMedicinal herbs
The invention relates to the field of traditional Chinese medicine modernization, in particular to a preparation method and a detection method of a Dipsacus standard decoction. The invention provides a preparation method of Dipsacus standard decoction, which comprises the following steps: taking the Dipsacus medicinal material, decocting it with water, and filtering to obtain a filtrate; Stage: a. Pre-freezing: Pre-freezing temperature is -50°C ~ -45°C; b. Primary drying: Drying temperature is -20°C ~ 0°C; c. Secondary drying: Drying temperature is 5 ~ 25°C, to obtain continuous Dipsacan standard decoction, the ointment rate of said Dipsacan standard decoction is 32.30-59.98%. After 6 months of accelerated stability test, the content is relatively stable, the moisture content is relatively stable, moisture absorption is resistant, and no impurity components are produced; the present invention Provides detection methods for the mass content, transfer rate, and characteristic spectrum of Dipsacus saponin VI, with good specificity, good repeatability, and good stability. It provides standards for the quality of traditional Chinese medicine formula granules, and realizes the quality control and effectiveness of traditional Chinese medicine formula granules. supervision.
Owner:GUOYAOJITUAN TONGJITANG (GUIZHOU) PHARMA CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com