Bushy knotweed leaf fingerprint HPLC method and its use in bushy knotweed leaf capsule quality control
A technology of fingerprint spectrum and Polygonum cuspidatum, which is applied to the HPLC method of Polygonum cuspidatum fingerprint and its application in the quality control of Polygonum cuspidatum capsules, can solve problems such as lack of scientific basis, achieve high separation of chromatographic peaks, and solve the problem of quality control. , the effect of stable retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Establishment of Polygonum cuspidatum Leaf Fingerprint HPLC Method
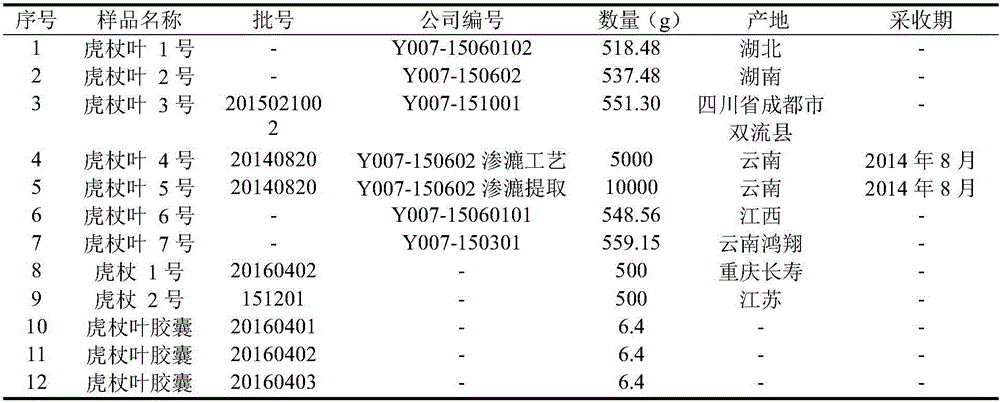
[0022] 1. Sample
[0023] Table 1 List of sample information
[0024]
[0025] 2. Reference substance
[0026] Table 2 List of reference substance information
[0027]
[0028] 2 sample preparation
[0029] 2.1 The medicinal material Polygonum cuspidatum No. 1-3, weigh 0.2500g to 100mL in a ground-mouth Erlenmeyer flask, add 20mL of 80% methanol, weigh, ultrasonicate (power 200W, frequency 40kHz) for 30min, make up the weight, and centrifuge for 10min. spare.
[0030] 2.2 Reference substance Take quercitrin reference substance, accurately weigh 1.73mg into a 10mL brown volumetric flask, add methanol to make a solution containing 0.173mg per mL, and obtain it. Take polydatin, resveratrol, quercetin and emodin reference substances, accurately weigh 5.70, 5.36, 5.39, and 5.61mg, put them into 25mL brown volumetric flasks, add methanol to make each mL contain 0.2280, 0.2144, 0.2156 ...
Embodiment 2
[0043] The UPLC-MS research of embodiment 2 Polygonum cuspidatum leaf medical material
[0044] 1. Sample
[0045] Polygonum cuspidatum No. 3 medicinal material sample.
[0046] 2. UPLC-MS conditions
[0047] 2.1 UPLC gradient conditions
[0048] Utilize Shimadzu Method Tranfer software to convert (column temperature: 20 ℃) into UPLC condition (column temperature: 20 ℃) in the HPLC method among the embodiment 1,
[0049] 2.2 Mass Spectrometry Conditions
[0050]
[0051]
[0052] 3. Results and Analysis
[0053] Through the UPLC-MS detection of Polygonum cuspidatum No. 3 sample, 20 components were preliminarily identified after analysis, as shown in Table 4.
[0054] Table 4 Retention time of main components, MS data and identification of compounds
[0055]
Embodiment 3
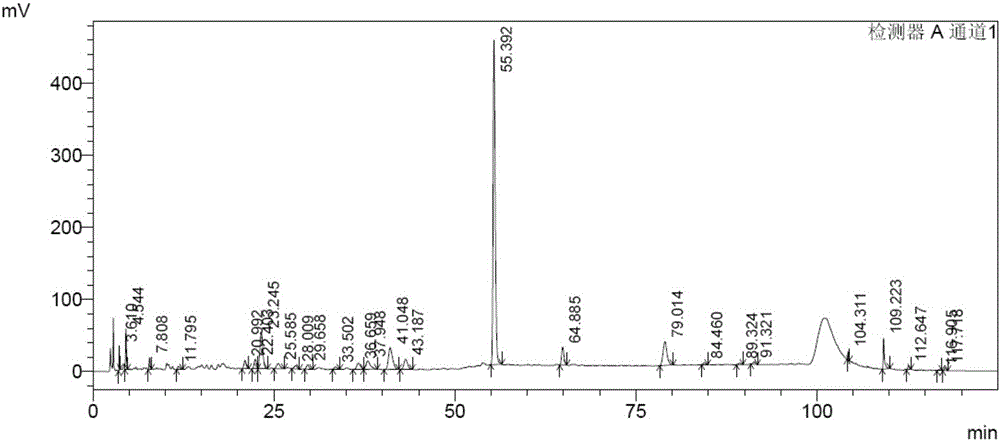
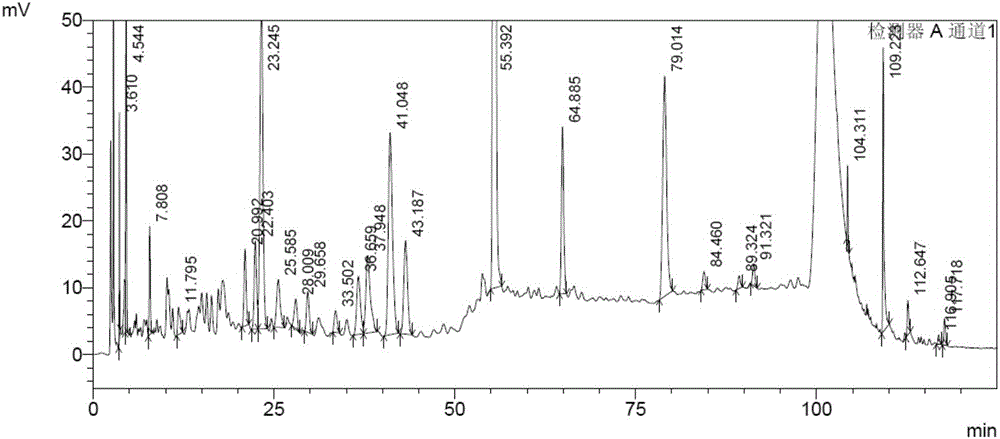
[0056] Example 3 Fingerprint Spectrum Research of Polygonum cuspidatum leaves and capsules
[0057] 1. Sample name
[0058] Fingerprint analysis samples include 6 batches of Polygonum cuspidatum leaves, 2 batches of Polygonum cuspidatum leaves and 3 batches of Polygonum cuspidatum capsules, see Table 1 in "Samples and Reference Substances in Example 1".
[0059] 2. Sample preparation
[0060] 2.1 The medicinal material is the medicinal material solution prepared in the optimization of HPLC conditions and the content determination in the content determination in Example 1.
[0061] 2.2 The finished product adopts the finished product solution prepared in “Optimization of HPLC Conditions in Example 1 and Content Determination in Content Determination”.
[0062] 3.3 Reference substance polydatin, quercetin, resveratrol, quercetin and emodin mixed reference solution, the concentrations are 0.0570, 0.0346, 0.0536, 0.0539, 0.0561mg / mL; the rest of the reference substances are diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com