Analysis method for determining releasing rate of quetiapine fumarate sustained release tablet
A technology for analyzing quetiapine fumarate and an analysis method, which is applied in the analysis field of determining the release rate of quetiapine fumarate sustained-release tablets, can solve problems such as failure to achieve basic release and drug inability to be released, and achieve stable retention time and peak good shape effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
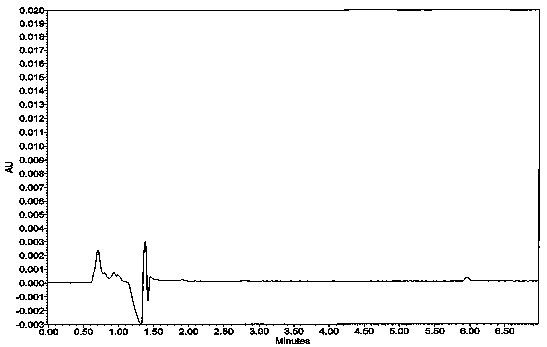
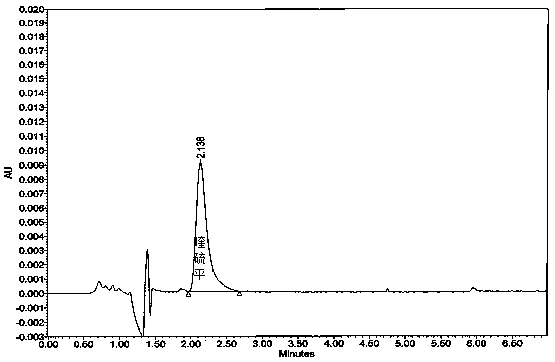
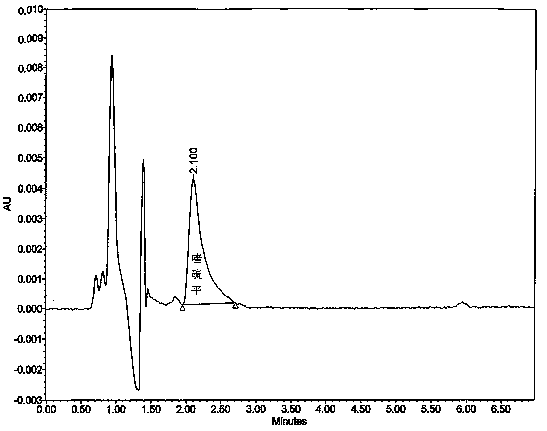
Image
Examples
Embodiment 1
[0026] Determination of the release rate of quetiapine fumarate sustained-release tablets (specification: 200mg, batch number: 19RD425001, containing 50% hyprolose K4M):
[0027] (1) Instruments and conditions:
[0028] Dissolution Apparatus: Agilent Dissolution Apparatus, USP Method 1, Blue Method;
[0029] Dissolution medium: pH6.8 buffer salt containing 1.0% sodium lauryl sulfate solution;
[0030] Medium volume: 500ml;
[0031] Speed: 100rpm;
[0032] Temperature: 37°C±0.5°C;
[0033] Sampling mode: automatic sampling;
[0034] Sampling volume: 10ml;
[0035] Sampling times: 1, 2, 4, 6, 9, 12, 16, 20 and 24 hours.
[0036] (2), experimental steps:
[0037] Take 6 tablets of this product, weigh and record the weight of each tablet, put them into each dissolution vessel respectively, and operate according to the law, respectively, at 1, 2, 4, 6, 9, 12, 16, 20 and 24 hours, from each dissolution vessel Remove the dissolution solution from the medium, filter the disso...
Embodiment 2
[0051] Determination of the release rate of quetiapine fumarate sustained-release tablets (specification: 200 mg, batch number: 19RD425002, containing 50% hyprolose K15M):
[0052] (1) Instruments and conditions:
[0053] Dissolution Apparatus: Agilent Dissolution Apparatus, USP Method 1, Blue Method;
[0054]Dissolution medium: pH6.8 buffer salt containing 1.0% sodium lauryl sulfate solution;
[0055] Medium volume: 500ml;
[0056] Speed: 100rpm;
[0057] Temperature: 37°C±0.5°C;
[0058] Sampling mode: automatic sampling;
[0059] Sampling volume: 10ml;
[0060] Sampling times: 1, 2, 4, 6, 9, 12, 16, 20 and 24 hours.
[0061] (2), experimental steps:
[0062] Take 6 tablets of this product, weigh and record the weight of each tablet, put them into each dissolution vessel respectively, and operate according to the law, respectively, at 1, 2, 4, 6, 9, 12, 16, 20 and 24 hours, from each dissolution vessel Remove the dissolution solution from the medium, filter the diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Medium volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com