Ophiopogon japonicus fingerprint, construction method thereof and ophiopogon japonicus quality detection method
A fingerprint and construction method technology, applied in the field of drug testing, can solve the problems of single quality evaluation method of Ophiopogon japonicus and indicate the overall quality of Ophiopogon japonicus and its preparations, and achieve stable retention time, no drift, and good reproducibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
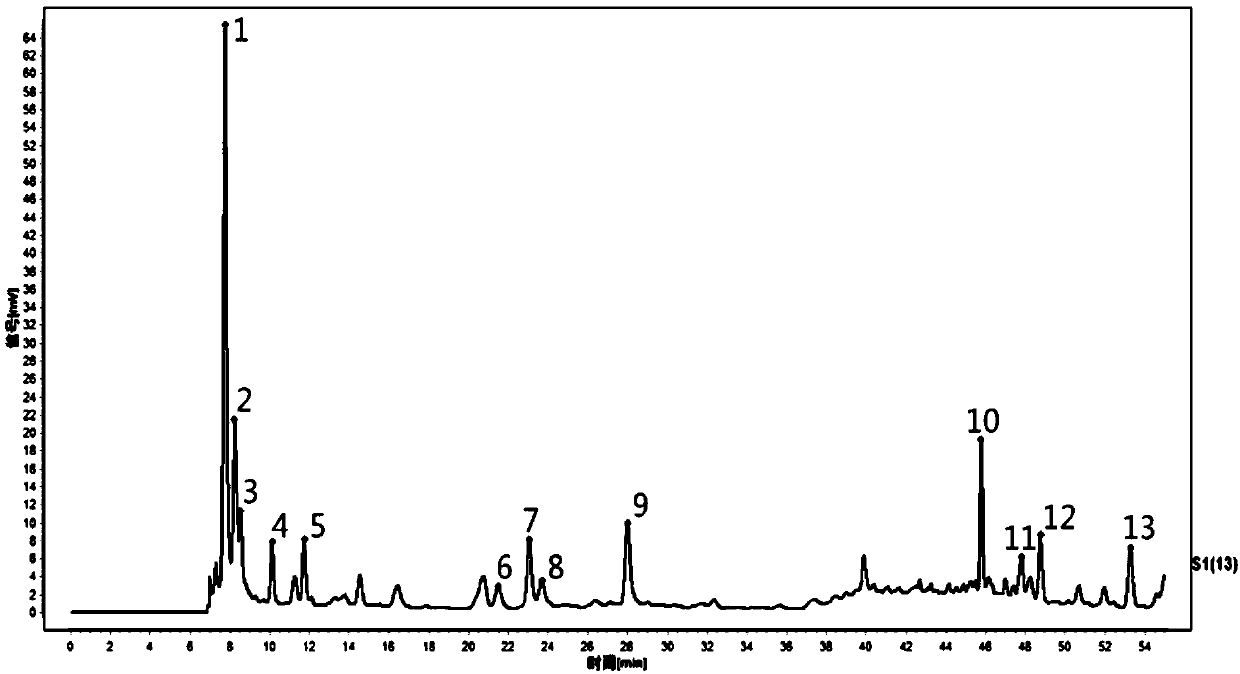
[0077] This embodiment provides a fingerprint of Ophiopogon japonicus, which includes the following steps for construction:
[0078] Prepare the test solution: accurately weigh 2.0 g of Ophiopogon japonicus, dry and crush; place in a stoppered conical flask, add 15 times the volume of water, weigh, decoct for 2 hours, cool, add weight, and filter to obtain the test product Solution.
[0079] Prepare the reference solution: accurately weigh an appropriate amount of the uridine reference substance, and use a 5% methanol aqueous solution to prepare a solution containing 50 μg uridine per 1 ml.
[0080] High performance liquid chromatography
[0081] According to high performance liquid chromatography (2015 edition of "Chinese Pharmacopoeia" three general rules 0512) test.
[0082] Chromatographic conditions: chromatographic column is Wters Bridge (5μm, 4.6×250mm Column); mobile phase: A: acetonitrile; B: 0.058% trifluoroacetic acid; gradient elution; column temperature: 40°C; detection wa...
Embodiment 2
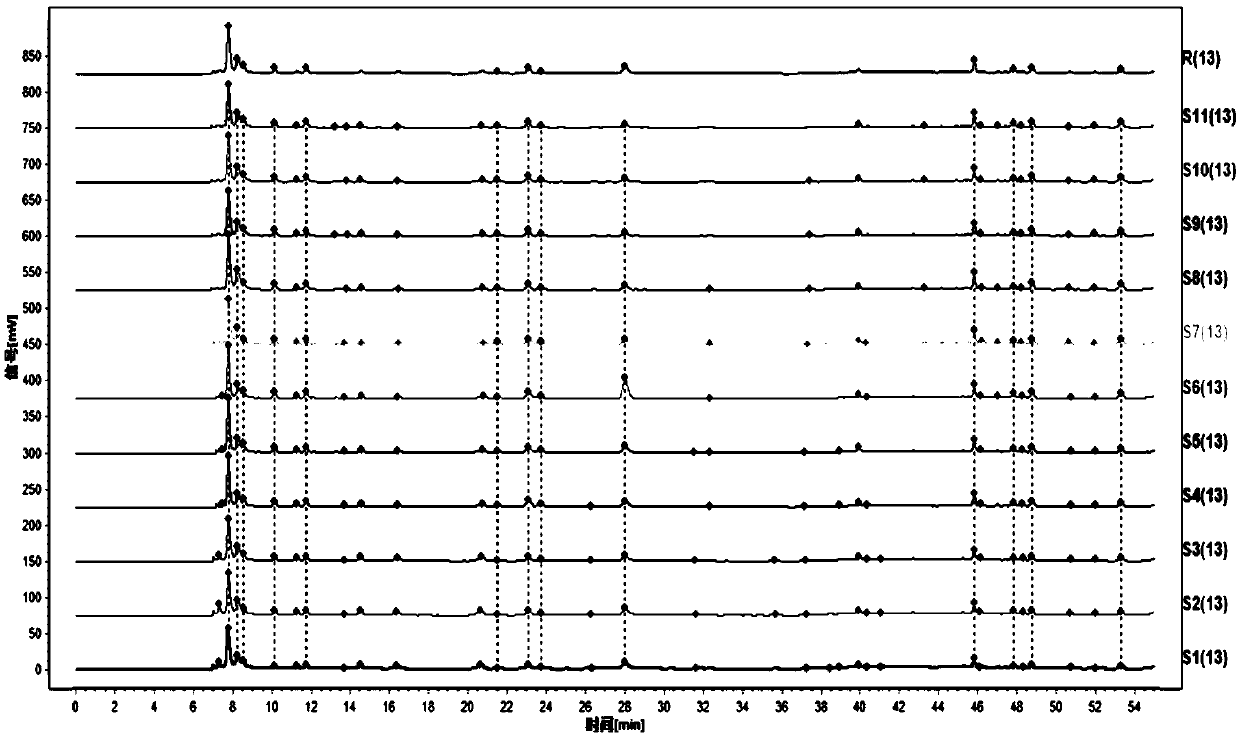
[0097] One of the differences between Example 2 and Example 1 is that the test solution used in Example 2 is different. In Example 2, the test solution is prepared by WS3-B-2865-98-2011 Shengmai injection Crafted.
[0098] Analyze the detection results of 11 batches of test product fingerprints with the similarity evaluation system of traditional Chinese medicine chromatographic fingerprints to generate control fingerprints; through the identification and identification of the characteristic peaks, 6 characteristic peaks of the HPLC fingerprint of the intermediate product of Ophiopogon japonicus are obtained , Fingerprints such as figure 2 As shown, with peak 2 uridine as the reference peak, the relative retention time of each characteristic peak is: peak 1: relative retention time is 0.859-0.868; relative peak area is 0.434-0.517; peak 2: relative retention time is 1; Relative peak area is 1; No. 3 peak: relative retention time is 1.235-1.242; relative peak area is 0.158-0.289;...
Embodiment 3
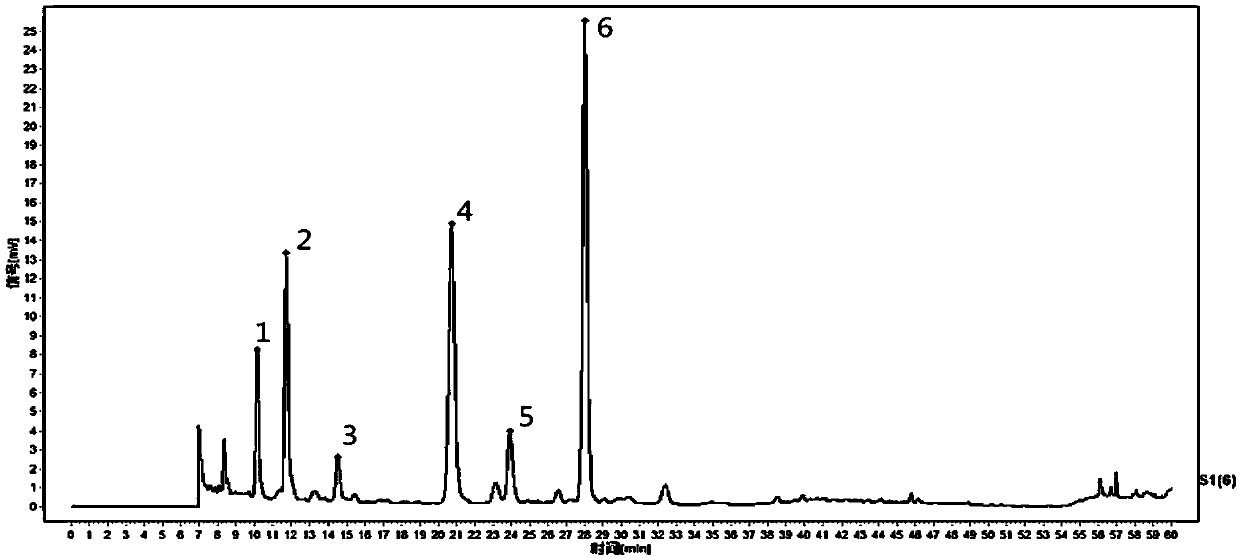
[0104] One of the differences between Example 3 and Example 1 is that the chromatographic conditions are different. The chromatographic conditions are: select the chromatographic column with octadecylsilane-bonded silica gel as the filler, use acetonitrile as the mobile phase A, and use 0.06vol% trifluoroacetic acid aqueous solution as the mobile phase B, respectively for the reference solution and the test solution Carry out gradient elution, and during the gradient elution process, the column temperature is controlled to 42° C., the flow rate is 0.61 mL / min; the detection wavelength is 278 nm.
[0105] The second difference between Example 3 and Example 1 is that the reference solution is different;
[0106] Reference solution: the solvent is 5 vol% methanol aqueous solution, the solute is uridine, and the uridine concentration is 51 μg / ml.
[0107] The third difference between Example 3 and Example 1 lies in the preparation of the test solution. In Example 3, the test solution wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com