Method for determining fingerprint spectrum of traditional Chinese medicine for treating coronary heart disease
A technology of traditional Chinese medicine preparations and fingerprints, applied in the field of medicine, can solve the problems of insufficient internal quality of traditional Chinese medicine preparations, and achieve the effects of improving neural function defects, good reproducibility, and inhibiting platelet aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
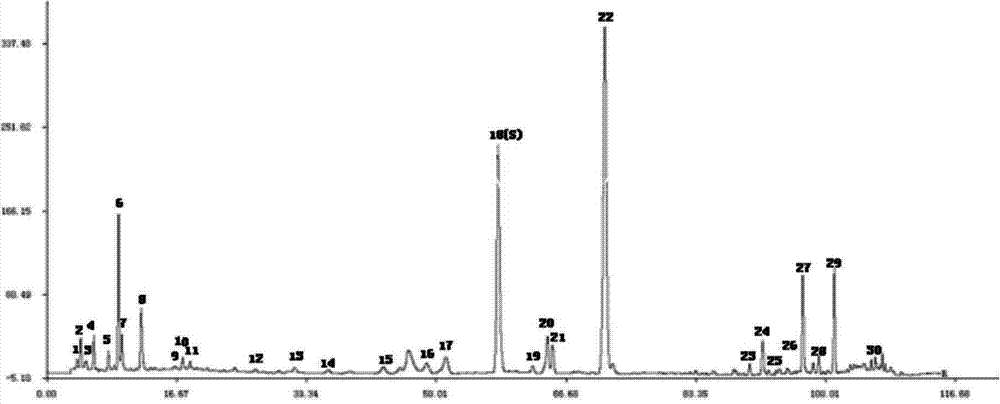
[0033] Example 1: Establishment of the fingerprint measurement method of the traditional Chinese medicine preparation of the present invention
[0034] 1.1 Instruments and reagents
[0035] Agilent1260 (quaternary pump G1311B; autosampler G1329B; column thermostat G1316A; diode array detector G4212B; LAB open chromatographic data workstation); electronic analytical balance (model BP211D, d=0.01mg, Germany Sartorius Balance Co., Ltd. ); Ultrasonic cleaner (model: KQ-250DE, Kunshan Ultrasonic Instrument Co., Ltd.); Agilent ZORBAX Extend-C 18 Chromatographic column (250mm×4.6mm, 5μm).
[0036] 1.2 Test drug
[0037] Ten batches of the Chinese medicine preparations of the present invention were produced by Shaanxi Buchang Pharmaceutical Co., Ltd., and the sample numbers of the ten batches were Y 1 , Y 2 , Y 3 , Y 4 , Y 5 , Y 6 , Y 7 , Y 8 , Y 9 , Y 10 , National Medicine Standard Z20020055, is a capsule. Methanol is chromatographically pure (Fisher Reagent Company, USA), water is Wahaha ...
Embodiment 2
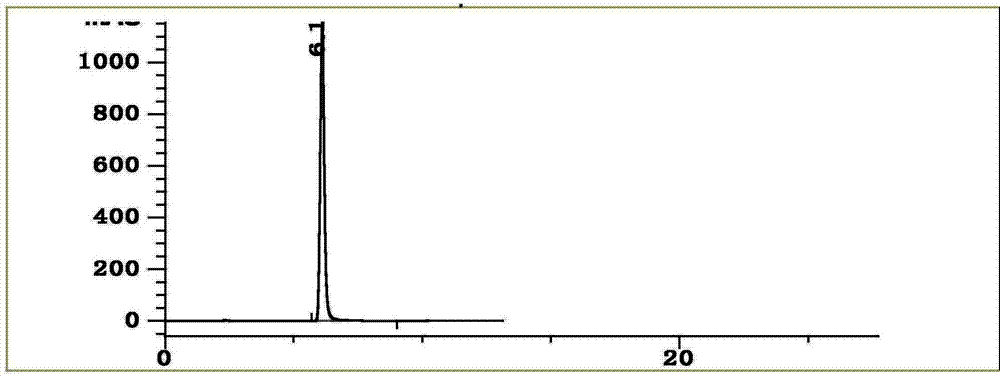
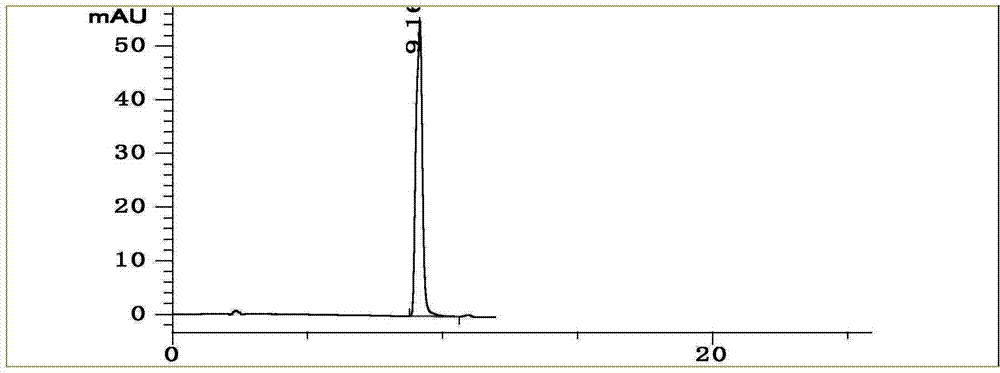
[0070] Example 2 Establishment of the fingerprint of the traditional Chinese medicine preparation of the present invention
[0071] 2.1 Preparation of test solution
[0072] Take about 1g of the contents of 10 different batches of traditional Chinese medicine preparations, accurately weigh them, place them in a 100mL conical flask with stopper, add 10mL of 70% methanol, weigh, ultrasonic (100W, 45kHz) for 30min, let cool, and make up with 70% methanol Lost weight, shake well, filter through 0.45μm microporous membrane, and take the filtrate to get it.
[0073] 2.2 Chromatographic conditions:
[0074] Column: Agilent ZORBAX Extend-C18 column (250mm×4.6mm, 5μm); column temperature: 35℃, flow rate: 1.0mL·min -1 , Use methanol as mobile phase A, 0.4% phosphoric acid aqueous solution as mobile phase B, and perform gradient elution according to the volume concentration ratio procedure in Table 5.
[0075] Table 5 Mobile phase gradient elution conditions
[0076]
[0077] Detection wavelength:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com