Synthetic method of ferrocene derivatives
A technology for ferrocene derivatives and synthesis methods, which is applied in the field of synthesis of ferrocene derivatives, and can solve the problems of inability to synthesize, poor universality of synthesis methods, low purity of monochloroferrocene, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
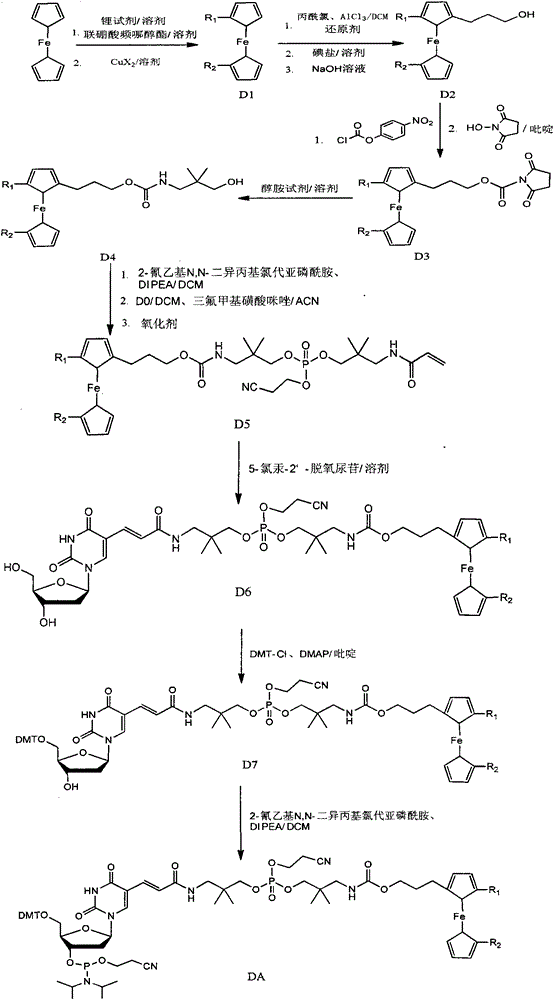
[0039] Example 1: Synthesis of DA1
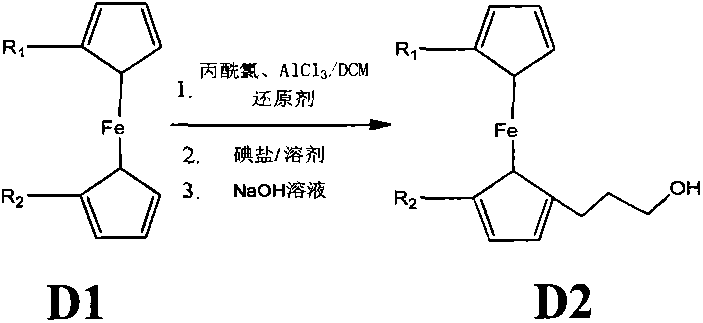
[0040] Synthesis of D21: Under anhydrous and anaerobic conditions, put 20g (107.5mmol) of ferrocene into a 2L round-bottom reaction flask and dissolve it with 200mL of anhydrous DCM, and add 15.4mL (161.3mmol) dropwise while stirring at 0°C. ) of 3-chloropropionyl chloride, drop it in 15 minutes, then add 21.5g (161mmol) anhydrous aluminum trichloride in batches, react for 2 hours, then add dropwise 215mL (215mmol, the concentration of borane in tetrahydrofuran is 1.0mol / L) borane tetrahydrofuran, the dropwise addition time is 1.5 hours, and after the dropwise reaction is completed for another 2 hours, it is quenched with ice water, washed, filtered, extracted, dried, and spin-dried. Dissolve the spin-dried substance with 200mL of N,N-dimethylformamide and 50mL of acetone, add 32.2g (214.7mmol) of NaI and react at 95°C for 3 hours, cool down to room temperature and oxidize with 60mL of 4mol / L hydrogen The sodium solution was hydrolyzed fo...
Embodiment 2
[0052] Embodiment 2: the synthesis of DA2
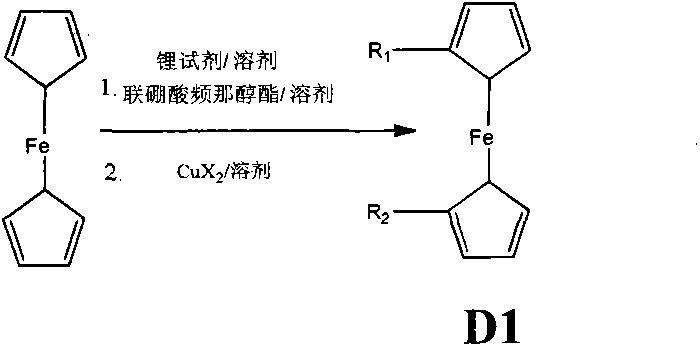
[0053] Synthesis of D12: Under anhydrous and oxygen-free conditions, put 60g (322.6mmol) ferrocene into the reaction flask and dissolve it with 300mL anhydrous tetrahydrofuran, add 372.2mL (1.3mol / L, 483.9mmol ) tert-butyllithium for 1 hour, warming up to room temperature for 2 hours, then cooling to -78°C, adding 122.9g (483.9mmol) biboronic acid pinacol ester for 1 hour, then naturally warming up to room temperature for 2 hours. The reaction was quenched with ice water, and then the quenched solution was washed, filtered, extracted, dried, and spin-dried, and silica gel was used as the filling phase, and the ferrocene was first rinsed with n-hexane to remove ferrocene, and then 25% DCM / Rinse with n-hexane, spin dry, and recrystallize to obtain pure ferrocene biboronic acid pinacol ester. 40g (128.2mmol) of the obtained pure ferrocene biboronic acid pinacol ester was dissolved in a 2000mL three-necked round-bottomed flask with 50m...
Embodiment 3
[0071] Embodiment 3: the synthesis of DA3
[0072] Synthesis of D13: Under anhydrous and anaerobic conditions, put 50g (268.8mmol) of ferrocene into a reaction flask and dissolve it in 250mL of anhydrous tetrahydrofuran, and add 252mL (n-butyllithium concentration of 1.6mol) at -5°C / L, 403.2mmol) n-butyllithium was reacted for 1 hour, heated to room temperature and reacted for 2 hours, then cooled to -78°C, added 101.6g (400mmol) pinacolate borate, reacted for 1 hour, and naturally heated to room temperature for reaction 2 Hour. The reaction was quenched with ice water, and then the quenched solution was washed, filtered, extracted, dried, and spin-dried, and silica gel was used as the filling phase. Rinse with methane / n-hexane, and finally rinse with 10% ethyl acetate / n-hexane, spin dry, and recrystallize until pure ferrocene disubstituted diboronic acid pinacol ester. The obtained pure ferrocene disubstituted diboronic acid pinacol ester 60g (137mmol) was dissolved in 200...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com