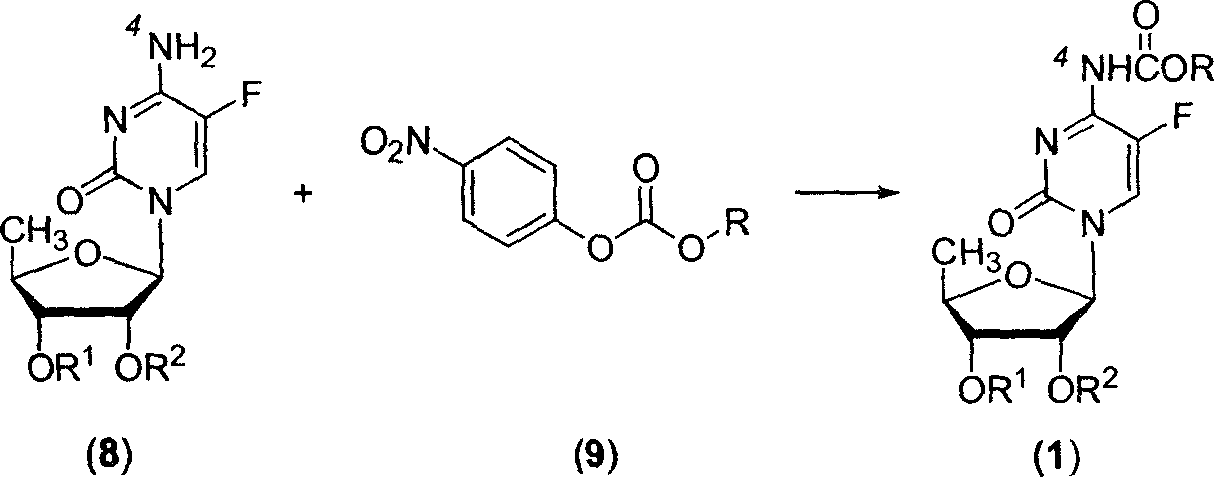
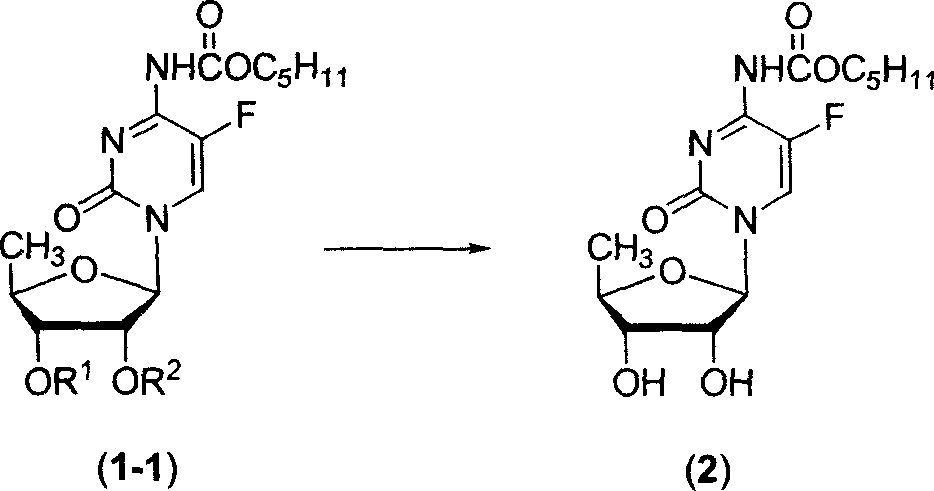
Fluoropyrimidine compound carbalkoxylation method
A compound and acetyl technology, applied in the field of synthesizing antitumor drug-capecitabine, can solve the problem of high device requirements, and achieve the effects of simple equipment, easy control and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
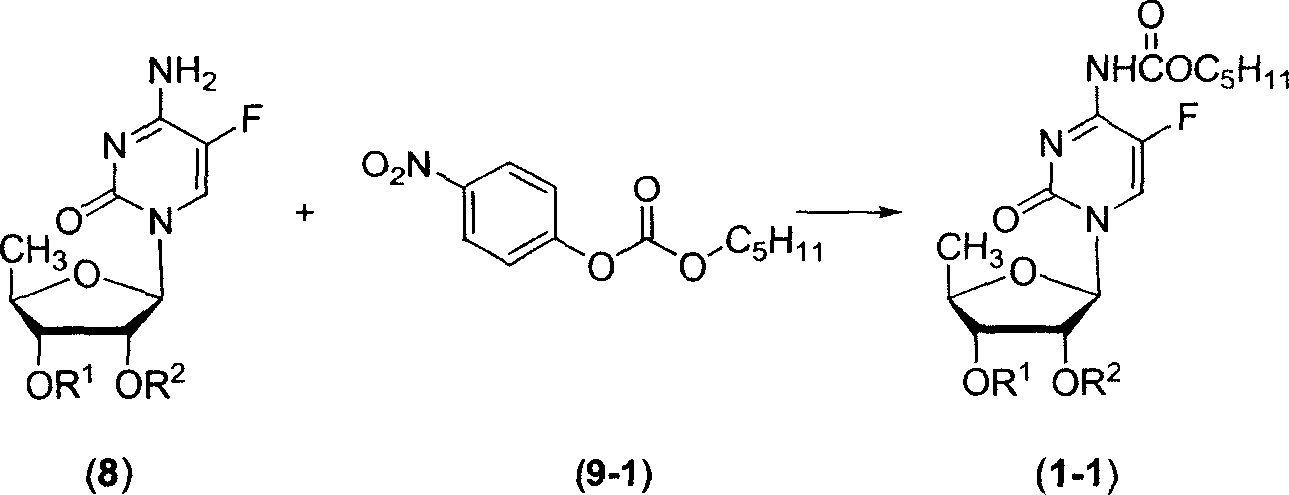
[0064] Example 1: 2', 3'-di-O-acetyl-5'-deoxy-5-fluoro-N 4 Preparation of -[(pentyloxy)carbonyl]cytidine nucleoside (1-1a)
[0065]
[0066] In a 100ml three-necked flask equipped with a thermometer, a reflux condenser, and mechanical stirring, add 2',3'-di-O-acetyl-5'-deoxy-5-fluoro-cytidine (8a) (3.29g, 10mmol ), a dry mixture of DMF and chloroform (50 mL, DMF:CHCl 3 =1:2, volume ratio) after stirring and dissolving, add pre-grinded potassium carbonate powder (4.83g, 35mmol), and n-pentoxyformyl p-nitrophenolate (9-1) (3.54g, 14mmol) , under stirring, heated to reflux for about 20 hours. After the reaction was completed, it was filtered, and the filter residue was washed with chloroform, and the organic phases were combined and concentrated under reduced pressure at 50° C. to obtain an oily substance. The oil was dissolved in ethyl acetate, washed with saturated brine, dried and desolvated, and the residue was purified by silica gel column chromatography to obtain 3.61...
Embodiment 2
[0068] Example 2: 2', 3'-di-O-acetyl-5'-deoxy-5-fluoro-N 4 - Preparation of [(butoxy)carbonyl]cytidine nucleoside (1-2a)
[0069]
[0070] According to the method of embodiment 1, replace n-pentoxyformyl p-nitrophenol ester (9-2) (40.8g, 0.24mol) in reference example 1 with p-nitrophenol n-butoxyformate 1), the other conditions were the same, and an oily substance was obtained. Purified by silica gel column chromatography to obtain 3.23 g of the title compound (1-2a) as an oil, with a yield of 75.3%.
[0071] 1 H NMR (400 MHz, DMSO-d6) δ10.61 (brs, 1H), 8.35 (brs, 1H), 5.84 (d, J=4.2Hz, 1H), 5.47 (br.t, 1H), 5.11 (br .t, 1H), 4.12(m, 3H), 2.08(s, 3H), 2.04(s, 3H), 1.59(m, 2H), 1.36(m, 5H), 0.86(t, J=7.2Hz, 3H) ppm.
Embodiment 3
[0072] Example 3: 2', 3'-isopropylidene-5'-deoxy-5-fluoro-N 4 - Preparation of [(pentyloxy)carbonyl]cytidine nucleoside (1-1b)
[0073]
[0074] In a 100ml three-neck flask equipped with a thermometer, a reflux condenser, and a magnetic stirrer, add 2',3'-isopropylidene-5'-deoxy-5-fluoro-cytidine (8b) (4.29g, 15mmol), n-pentyloxyformyl p-nitrophenolate (9-1) (4.30g, 17mmol), dried DMF (50mL) was stirred and dissolved, then added pre-ground potassium carbonate powder (4.83g, 35mmol) and stirred, React at 35°C for about 25 hours. After the reaction was completed, it was filtered, and the filter residue was washed with ethyl acetate, and the organic phases were combined and concentrated under reduced pressure at 50° C. to obtain an oily substance. The oil was dissolved in ethyl acetate, washed with saturated brine, dried, and desolvated. The residue was purified by silica gel column chromatography to obtain 5.11 g of the title compound (1-1b) as a foam, with a yield of 85.3%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com