Method for preparing stable albumin nano-particles by virtue of thermal denaturation
A technology of nano-albumin and albumin, which is applied in the direction of pharmaceutical formulations, organic active ingredients, and medical preparations containing active ingredients, etc. It can solve the problems of unfavorable production quality stability, and the inability of nanoparticles to withstand the dilution of buffer solutions and serum albumin solutions , endangering the life safety of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1, preparation of stable bovine serum albumin nanoparticles.
[0036] Using bovine serum albumin and vanillin as raw materials, bovine serum albumin nanoparticles stabilized by disulfide bonds and amide bonds were prepared.
[0037] The preparation method is as follows:
[0038] Dissolve 250 mg of bovine serum albumin and 14.4 mg of vanillin in 50 mL of purified water, heat at 70 °C for 2 h and then cool to room temperature, then place the prepared nanoparticle solution in a dialysis bag and dialyze in purified water at 4 °C for 24 h. That is, stable bovine serum albumin nanoparticles are obtained, and the average particle diameter of the albumin nanoparticles is 50-300 nm.
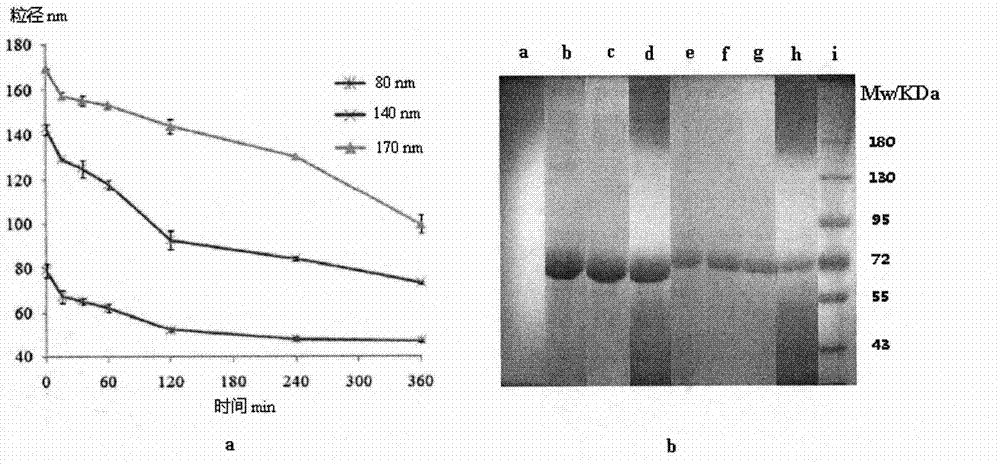
[0039] The stepwise degradation process of the prepared nanoparticles in trypsin solution can be obtained from figure 1 The particle size change curve of a and figure 1 It is confirmed in the SDS-PAGE gel electrophoresis of b.
[0040] The stepwise degradation process of the prepared nano...
Embodiment 2
[0041] Example 2, preparing stable bovine serum albumin nanoparticles.
[0042] Using bovine serum albumin and vanillin as raw materials, bovine serum albumin nanoparticles stabilized by disulfide bonds and amide bonds were prepared.
[0043] The preparation method is as follows:
[0044] Dissolve 250 mg of bovine serum albumin and 14.4 mg of vanillin in 50 mL of purified water, heat at 80°C for 1 hour and cool to room temperature, then place the prepared nanoparticle solution in a dialysis bag and dialyze in purified water at 4°C for 24 hours. That is, stable bovine serum albumin nanoparticles are obtained, and the average particle diameter of the albumin nanoparticles is 50-500 nm.
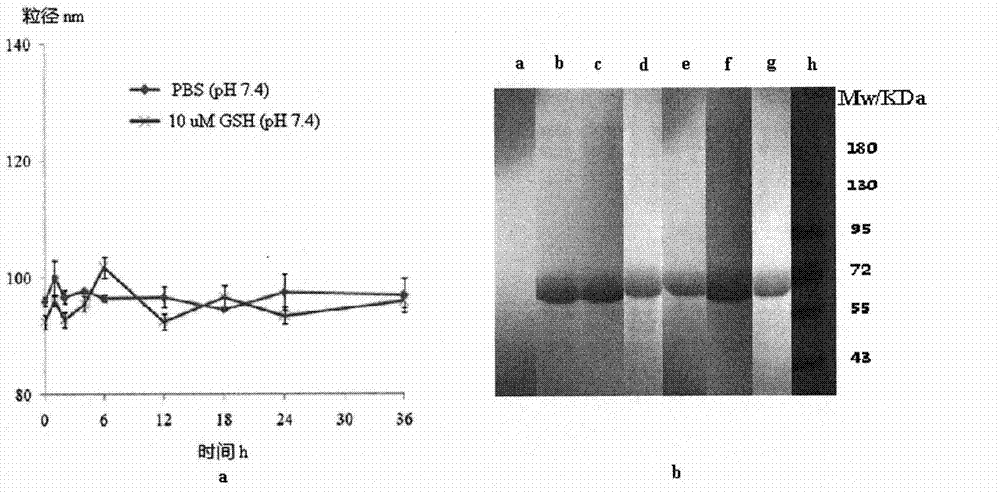
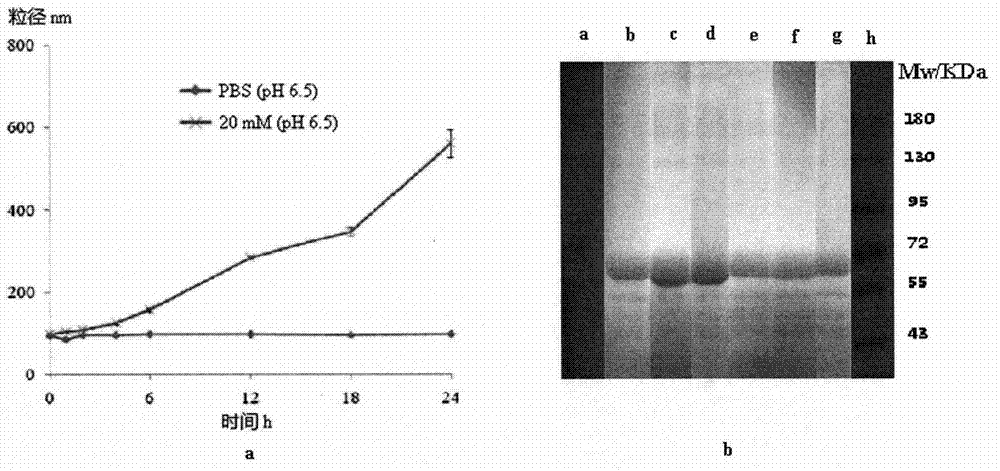
[0045] The stability of the resulting albumin nanoparticles stored in phosphate buffered saline at 4°C can be obtained from Figure 4 look out.
[0046] The change of the free sulfhydryl groups of the obtained albumin nanoparticles during the preparation process can be obtained from Figure ...
Embodiment 3
[0049] Example 3, preparing a stable doxorubicin hydrochloride-human serum albumin nanoparticle drug delivery system.
[0050] Using human serum albumin, doxorubicin hydrochloride and vanillin as raw materials, a drug delivery system of human serum albumin nanoparticles loaded with doxorubicin hydrochloride stabilized by disulfide bonds and amide bonds was prepared.
[0051] The preparation method is as follows:
[0052] Dissolve 250 mg of human serum albumin and 14.4 mg of vanillin in 50 mL of purified water, and heat at a constant temperature of 70° C. for 2 hours to obtain a human serum albumin nanoparticle solution. After the above-mentioned human serum albumin nanoparticle solution was cooled to room temperature, 2.5 mL of a 5 mg / mL doxorubicin hydrochloride solution was heated therein, and placed overnight in the dark. The obtained nano-suspension of human serum albumin was placed in a dialysis bag, and dialyzed in purified water at 4° C. for 24 hours to obtain human se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| electric potential / voltage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com