A kind of poly(beta-amino ester) type polymer gene carrier containing disulfide bond and its synthesis method and application
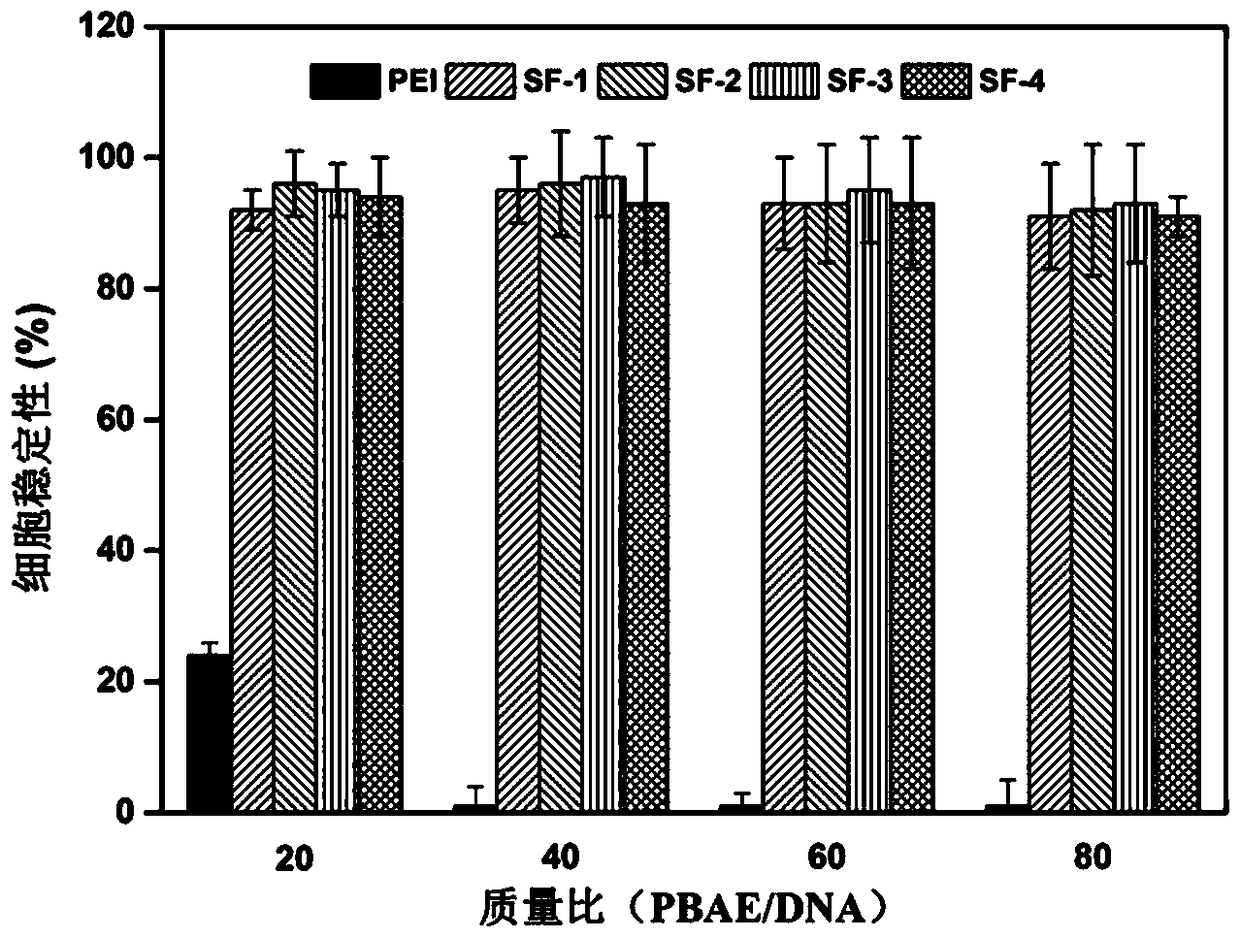
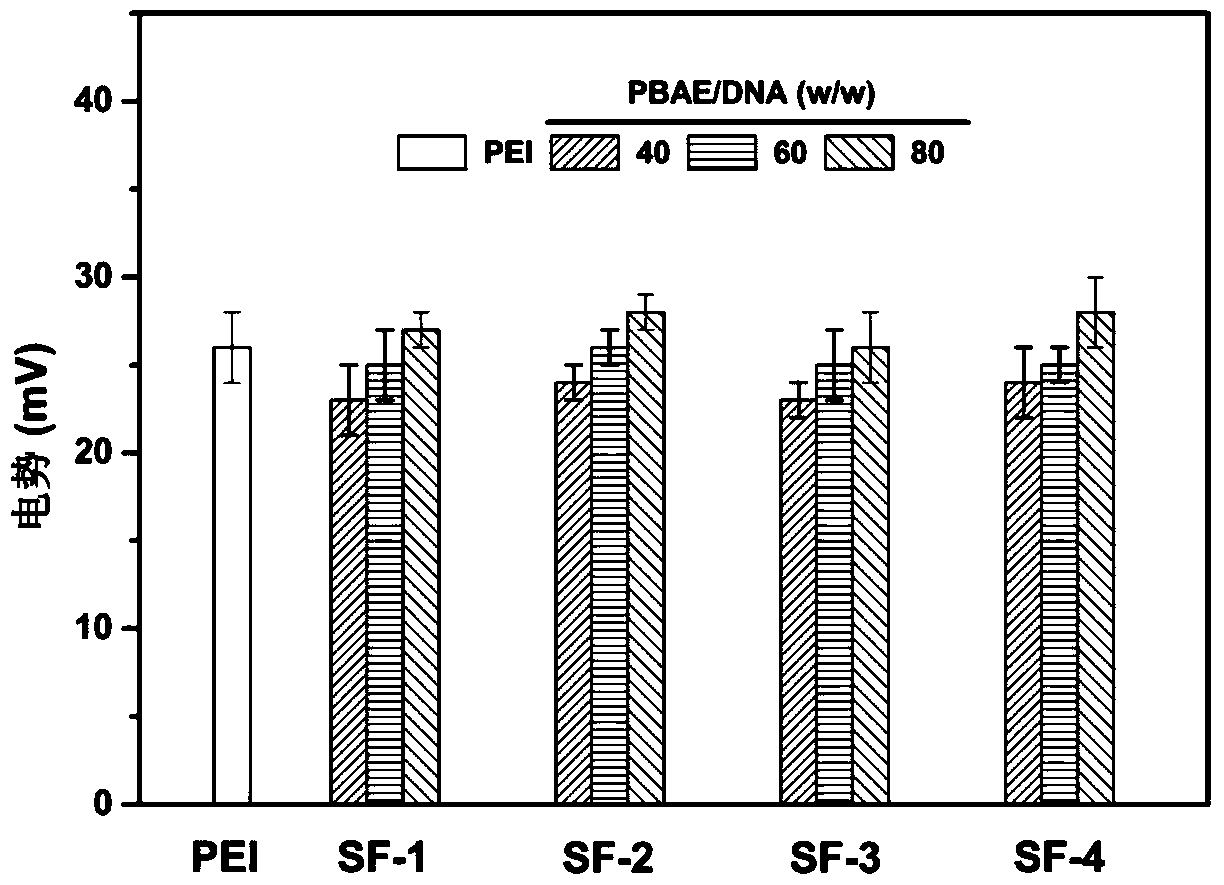
A technology of gene carrier and amino ester, which is applied in the field of cluster polymer non-viral gene carrier and its synthesis, can solve the problems of inability to fully release DNA in cells and increase cytotoxicity, and achieve improved transfection effect and cytotoxicity Effects of reducing and increasing transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
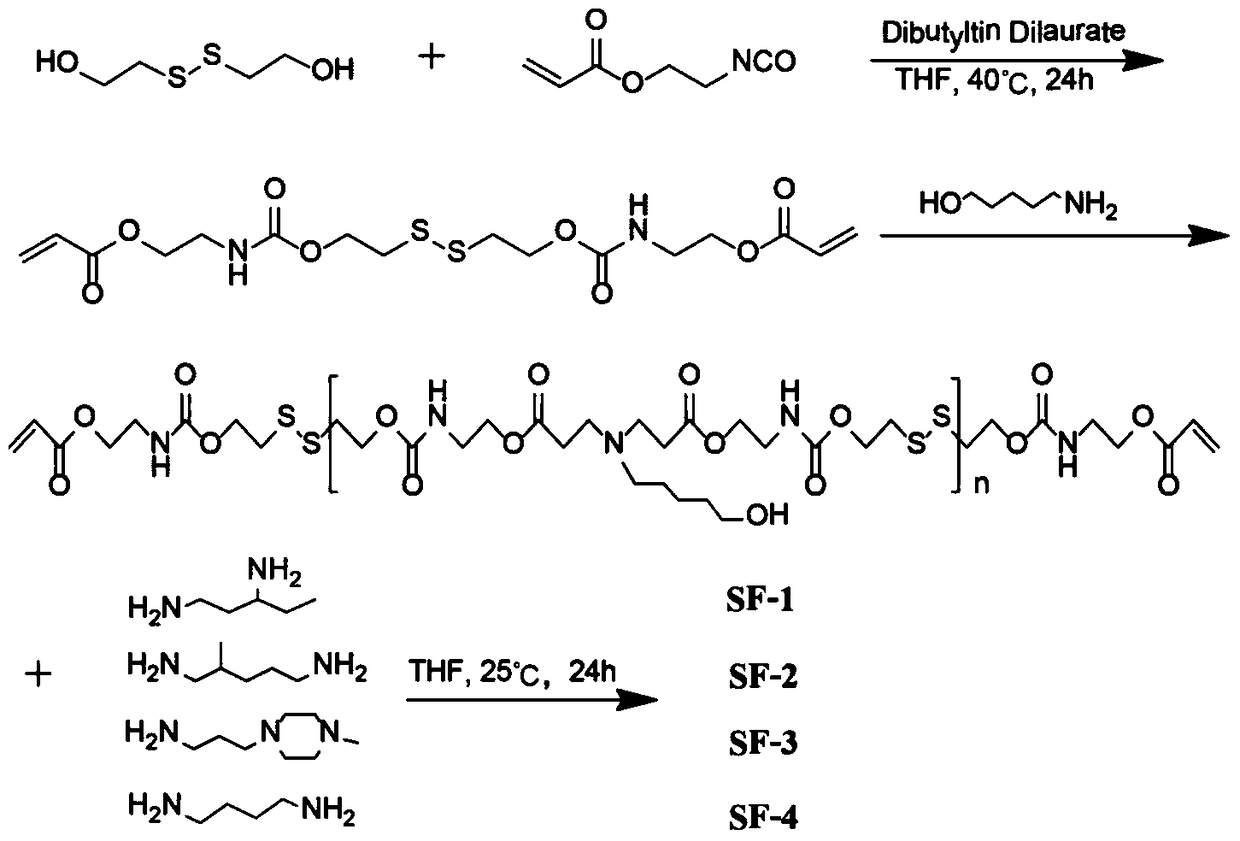
[0038] Step 1. Dissolve 2-hydroxyethyl disulfide (3mmol) in THF solution (10mL), add isocyanoethyl acrylate (6mmol), add dropwise two drops of dibutyltin dilaurate (catalyst), at 40°C The reaction was stirred for 24h. After the reaction, the mixed solution was rotary evaporated to obtain a crude product, which was then purified by column chromatography (ethyl acetate / petroleum ether=1 / 1).
[0039] S (yield 95%): 1 H NMR (500MHz, DMSO) δ7.37 (t, J = 5.6Hz, 2H), 6.33 (dd, J = 17.3, 1.3Hz, 2H), 6.14 (dd, J = 17.3, 10.4Hz, 2H), 5.93 (dd,J=10.4,1.2Hz,2H),4.16(t,J=6.3Hz,4H),4.08(t,J=5.5Hz,4H),3.24(q,J=5.6Hz,4H),2.92 (t, J=6.3Hz, 4H).
[0040]Step 2, the diacrylate monomer compound S (2.2 mmol) synthesized in step 1 and 5-amino-1-pentanol (2 mmol) were stirred and reacted for 24 hours without solvent, and the reaction temperature was set at 70 ° C to generate acrylic acid Ester terminated poly(β-amino ester) SF. SF (yield 100%): 1 H NMR (500MHz, DMSO) δ6.35 (CH 2 =CH-), 6.14 (...
Embodiment 2
[0043] The difference between embodiment 2 and embodiment 1 is step 2, and step 1 and step 3 are the same as embodiment 1.
[0044] Step 2, the diacrylate monomer compound S (2.2 mmol) synthesized in step 1 and 5-amino-1-pentanol (2 mmol) were stirred and reacted for 48 hours without solvent, and the reaction temperature was set at 70 ° C to generate acrylic acid Ester terminated poly(β-amino ester) SF.
Embodiment 3
[0046] The difference between embodiment 3 and embodiment 1 is step 2, and step 1 and step 3 are the same as embodiment 1.
[0047] Step 2, the diacrylate monomer compound S (2.2 mmol) synthesized in step 1 and 5-amino-1-pentanol (2 mmol) were stirred and reacted for 24 hours without solvent, and the reaction temperature was set at 90 ° C to generate acrylic acid Ester terminated poly(β-amino ester) SF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com