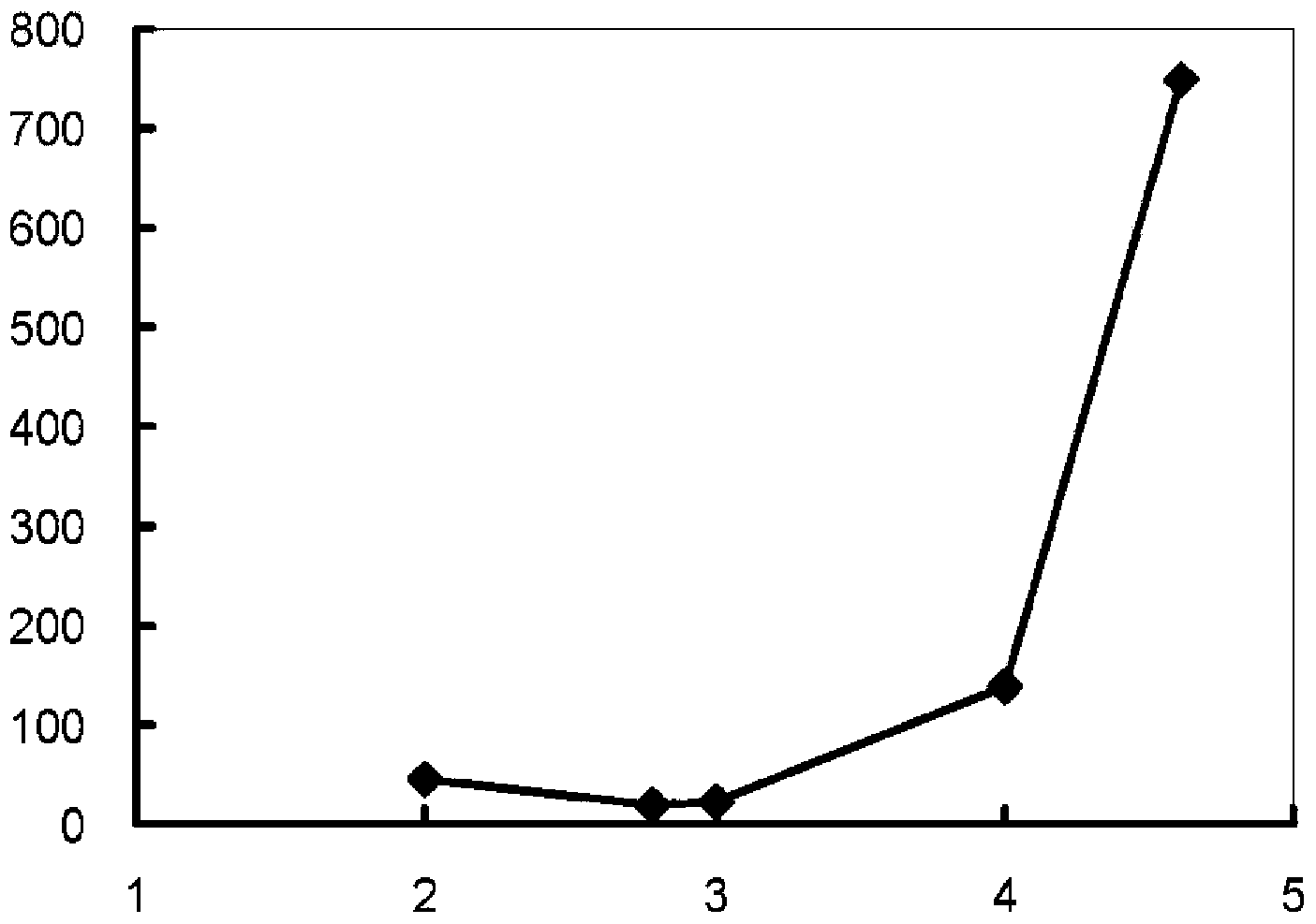
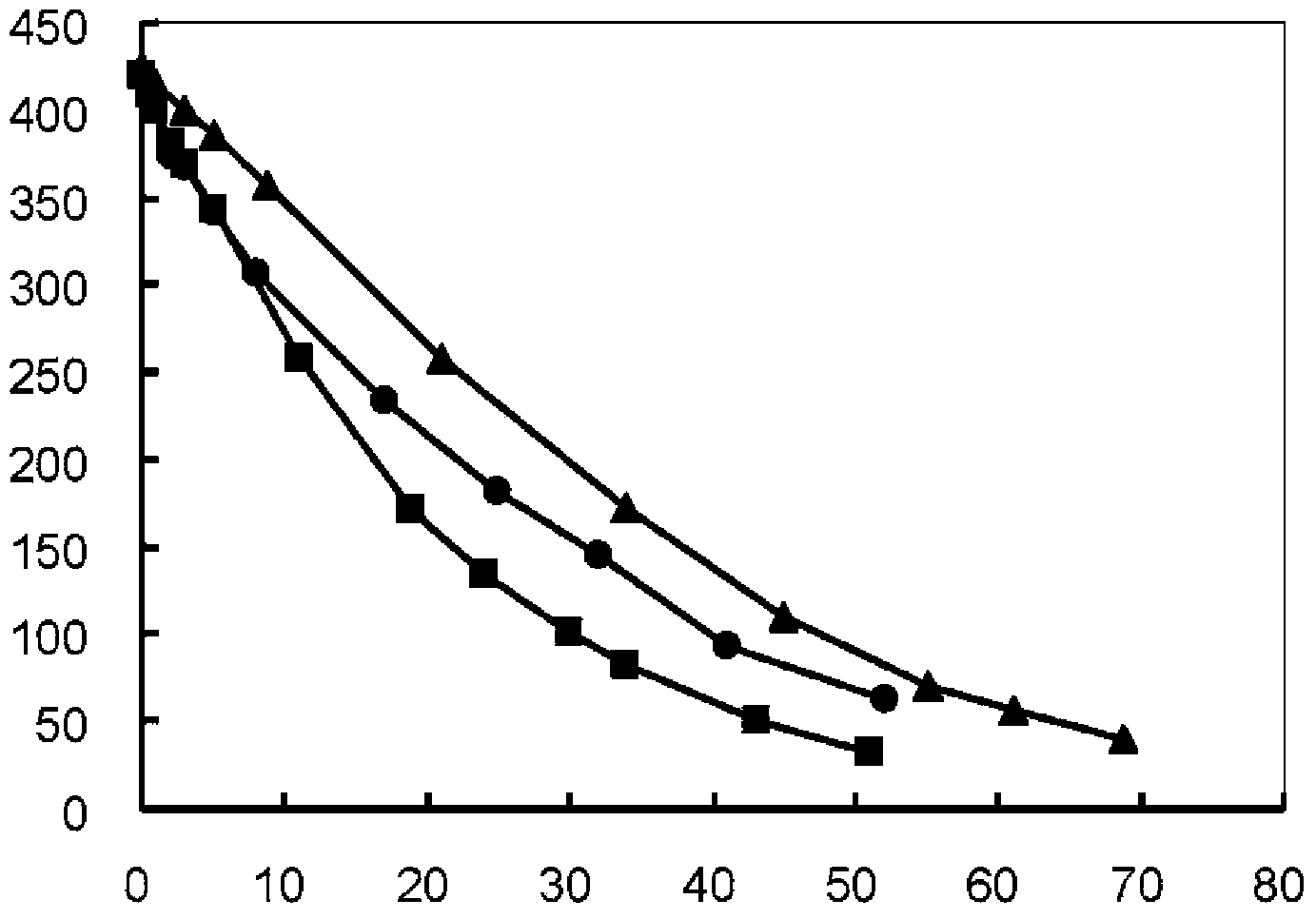
Process for producing reduced glutathione
一种氧化型谷胱甘肽、谷胱甘肽的技术,应用在肽的制备方法、化学仪器和方法、肽等方向,能够解决谷胱甘肽晶体阻塞电解槽等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Electrolytic Reduction of Oxidized Glutathione (1)
[0051] Sodium hydroxide was added to the oxidized glutathione aqueous solution to adjust the pH to 6.5, thereby preparing a 400 g / L oxidized glutathione aqueous solution. As the electrolytic cell, an anode cell (15L) and a cathode cell (15L) are used, and the effective membrane area is 1.8dm 2 A cation exchange membrane SELEMION CMT (manufactured by Asahi Glass Co., Ltd.) separates the two tanks. A platinized titanium plate was used as the anode, and a zinc plate was used as the cathode. The distance between the electrodes and the cation exchange membrane was set to 1.9 mm, and the circulation flow rate was set to 240 L / hour. The anode tank contained a 0.50 mol / L sulfuric acid solution (5 L), and the cathode tank contained the aqueous oxidized glutathione solution (400 g / L, 5 L) prepared above.
[0052] At electrolysis voltage 6V, electrolysis current 6.6-8.3A / dm 2 And the electrolytic reduction reaction is carrie...
Embodiment 2
[0055] Electrolytic Reduction of Oxidized Glutathione (2)
[0056] Sodium hydroxide was added to adjust the pH of the solution to 4.5, thereby preparing a 400 g / L oxidized glutathione aqueous solution. Electrolytic reduction was performed under the same conditions as in Example 1, whereby it was confirmed that 1.73 kg of reduced glutathione was produced within 52 hours (conversion rate 86.7%).
[0057] The amount of zinc ions in the cathode tank solution after completion of electrolysis was confirmed in the same manner, thereby confirming the dissolution of 430 mg of zinc.
Embodiment 3
[0059] Electrolytic Reduction of Oxidized Glutathione (3)
[0060] Use graphite electrode as cathode and use the oxidized glutathione solution under the same conditions as in Example 1, at electrolysis voltage 6V, electrolysis current 6.6-8.3A / dm 2 electrolytic reduction reaction at room temperature. The resulting product in the cathode cell was quantified by HPLC under the same conditions as in Example 1, and it was confirmed that 1.82 kg of reduced glutathione was produced within 70 hours (conversion rate 90.7%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com