Fasudil salt injection for improving stability and preparation method thereof
A technology of fasudil and injection, which is applied in the field of fasudil salt injection preparations, can solve the problems of not considering the unstable factors of drug ingredients, achieve the effect of large industrial application value and prolong the shelf life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
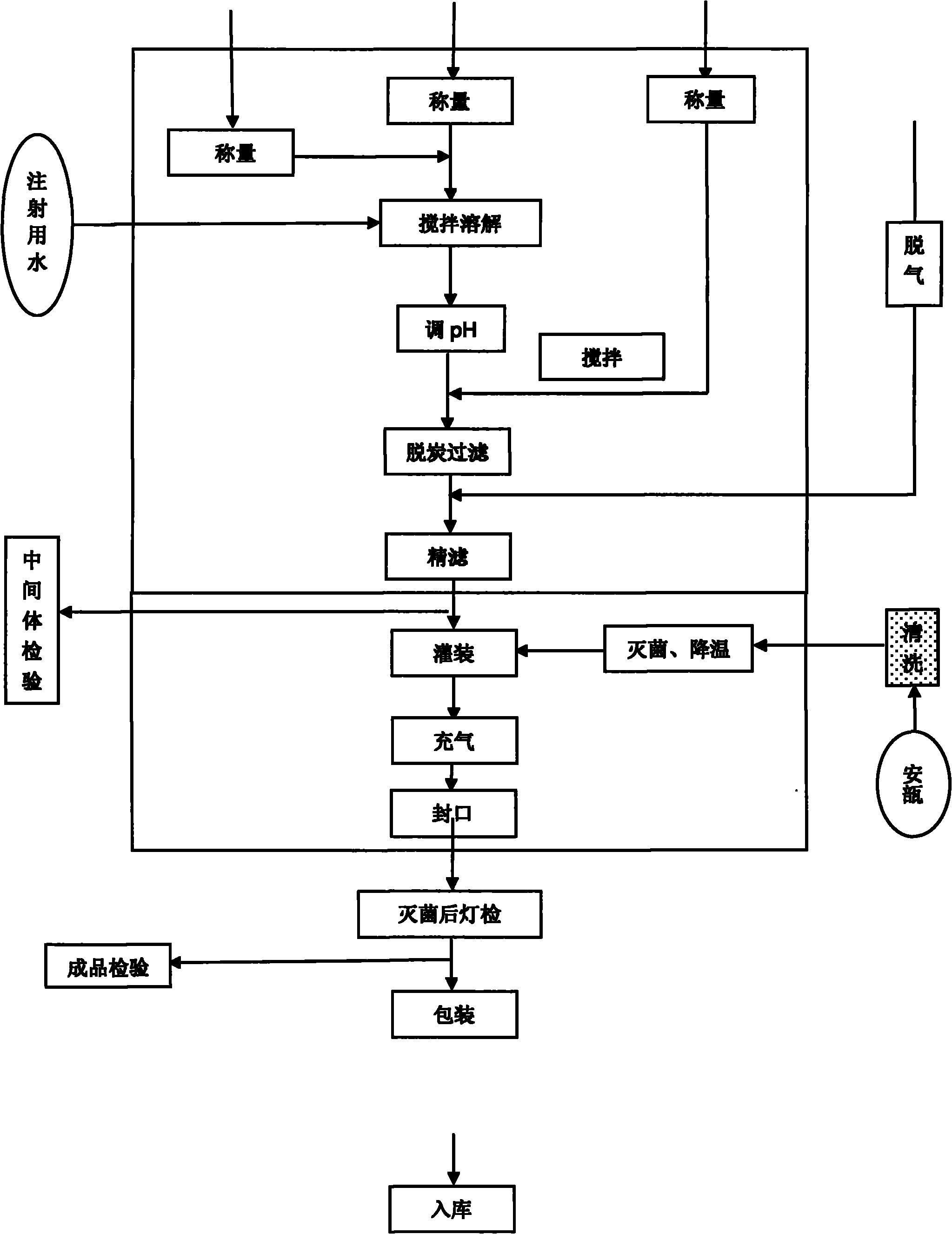
[0028] Embodiment 1 prepares Fasudil hydrochloride injection
[0029] Get 30.0g of Fasudil hydrochloride, 10.0g of medicinal sodium chloride, and water for injection to 2000ml, and make 1000 sticks altogether, that is, each stick is 30mg / 2ml.
[0030] The preparation method is as follows:
[0031] 1. Bottle washing: The bottle washing post is a clean area, the cleanliness should meet the 100,000-level standard, and the bottle area has 100-level laminar flow protection. The glass ampoules for injection are sorted and enter the ultrasonic bottle washing machine, washed with purified water in turn, dried with compressed air, washed with water for injection, and dried with compressed air. The outer body of the bottle is sprayed, cleaned and dried with circulating water, water for injection and compressed air in order to complete the whole cleaning process.
[0032] 2. Tunnel oven sterilization: The air cleanliness of the cleaned glass ampoules for injection (infrared) room shoul...
Embodiment 2
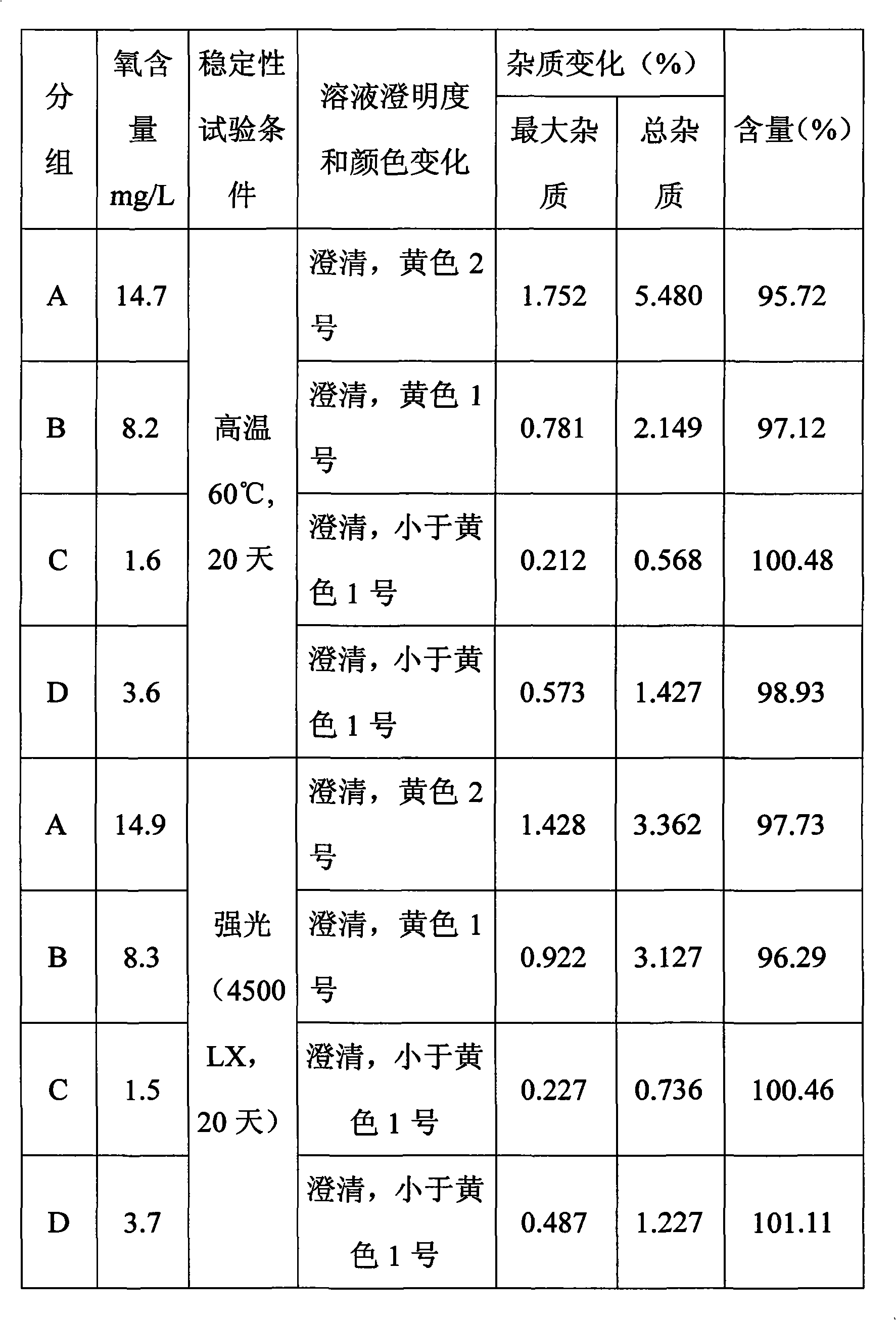
[0037] Embodiment 2, the impact of oxygen content in the injection on the stability of the preparation
[0038] In order to test the effect of the oxygen content in Fasudil Hydrochloride Injection on the stability of the injection, injections with different oxygen contents were first prepared, and then the changes of impurity content in the injection were detected under different high temperature and light conditions.
[0039] The experiment was carried out in the following way:
[0040] 1, the preparation of different oxygen content solutions of the test: first prepare the solution that is used for the production of injection according to embodiment 1 fasudil hydrochloride injection, then adopt the method for ultrasound and negative pressure pumping to remove the gas in the injection, Oxygen is introduced to increase the oxygen content in the solution to prepare injections with different oxygen content.
[0041] The following injections with different oxygen concentrations wer...
Embodiment 3
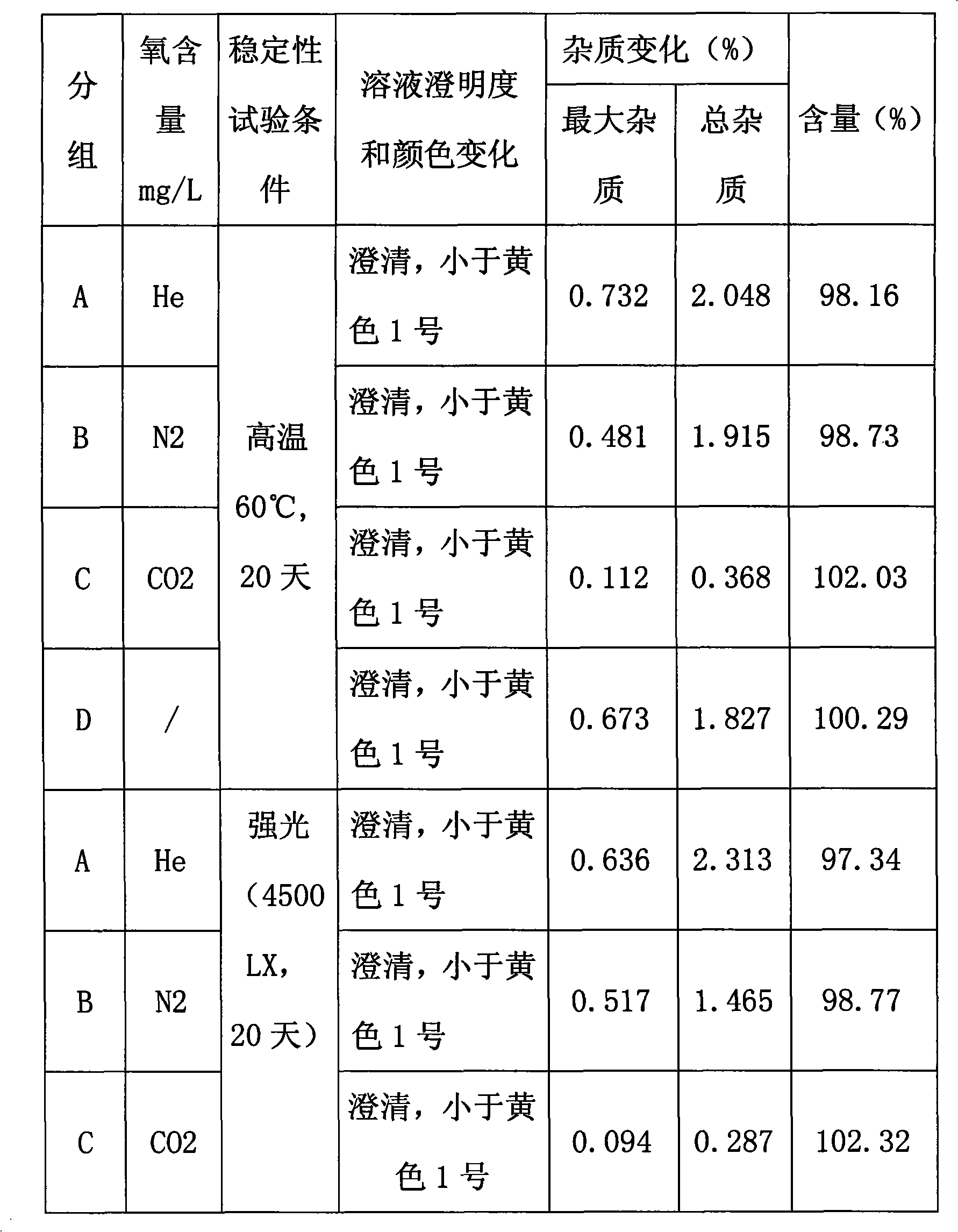
[0048] Embodiment 3, the impact on the stability of Fasudil hydrochloride injection preparation after being filled with inert gas
[0049] In order to test the influence of inert gas such as nitrogen, helium, carbon dioxide gas on the stability of injection in the Fasudil Hydrochloride Injection, at first the Fasudil Hydrochloride Injection of Embodiment 1 is carried out ultrasound or / and pumping Negative pressure degassing, and then injecting different gases to prepare injections containing different gases (nitrogen, helium or carbon dioxide gas), and then detecting the change of impurity content in the injections under different high temperature and light conditions.
[0050] The experiment was carried out in the following way:
[0051] 1. Preparation of solutions containing different inert gases (helium, nitrogen, carbon dioxide): first prepare a solution for the production of injections according to Example 1 Fasudil Hydrochloride Injection. Then, the gas in the injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com