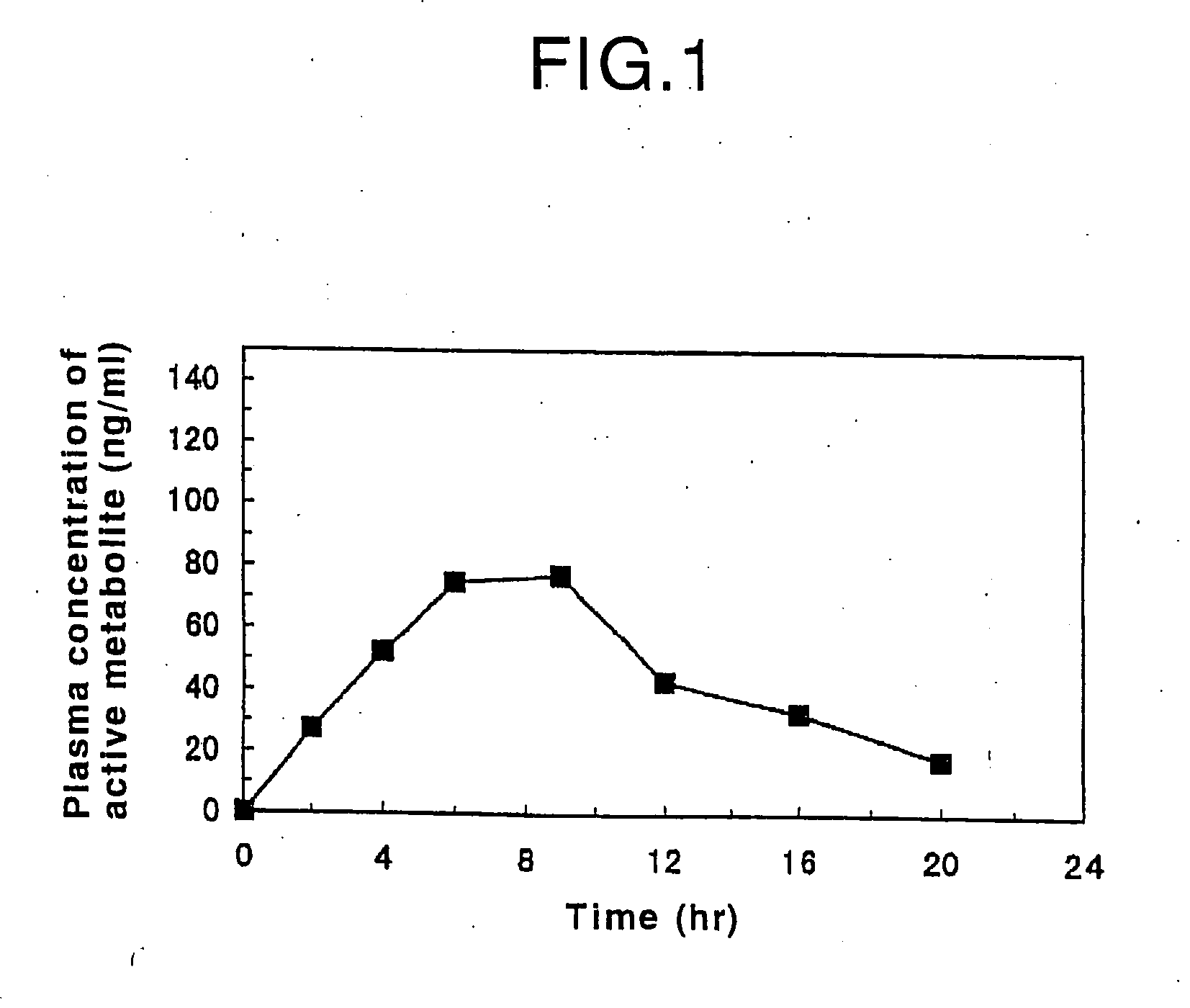
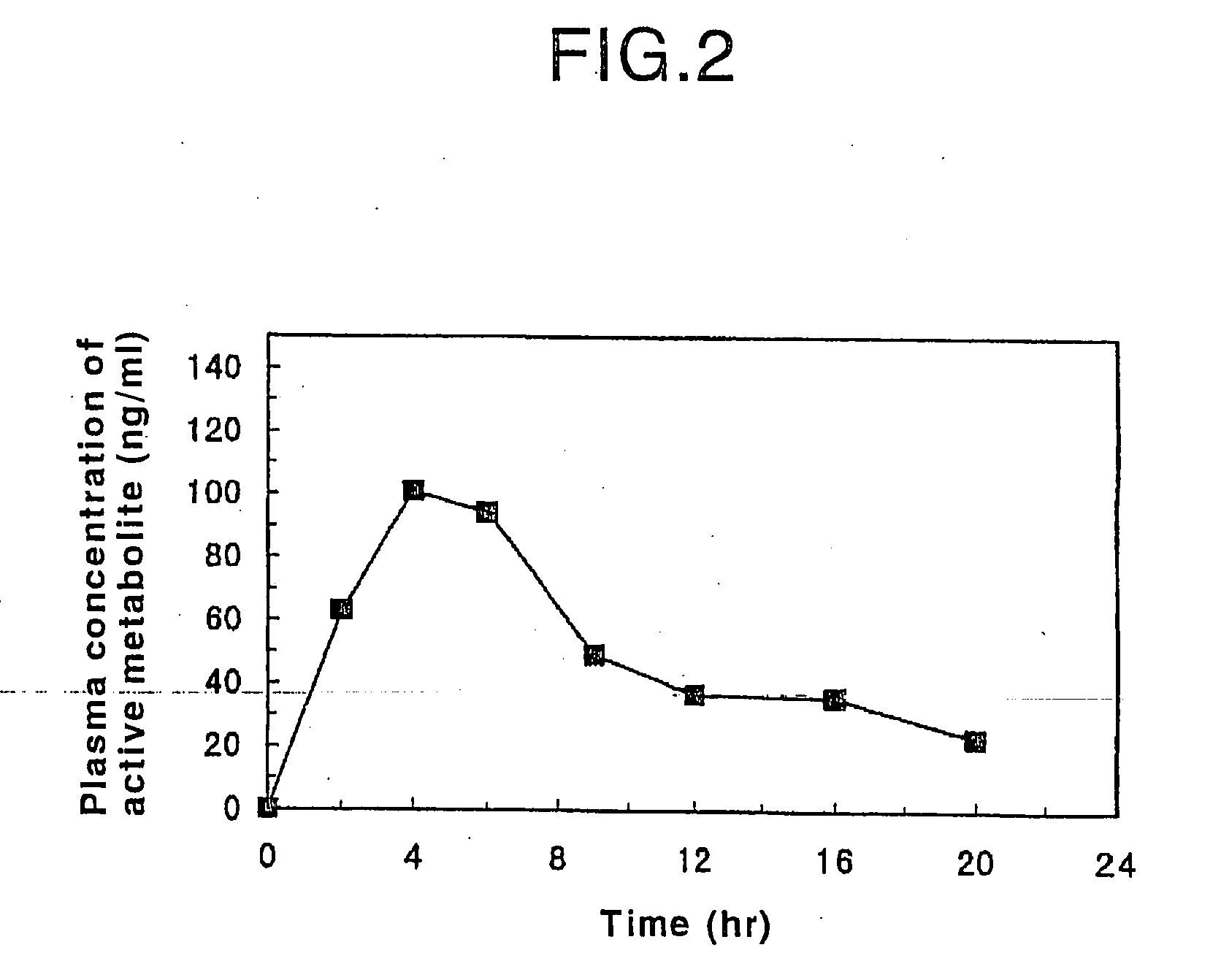
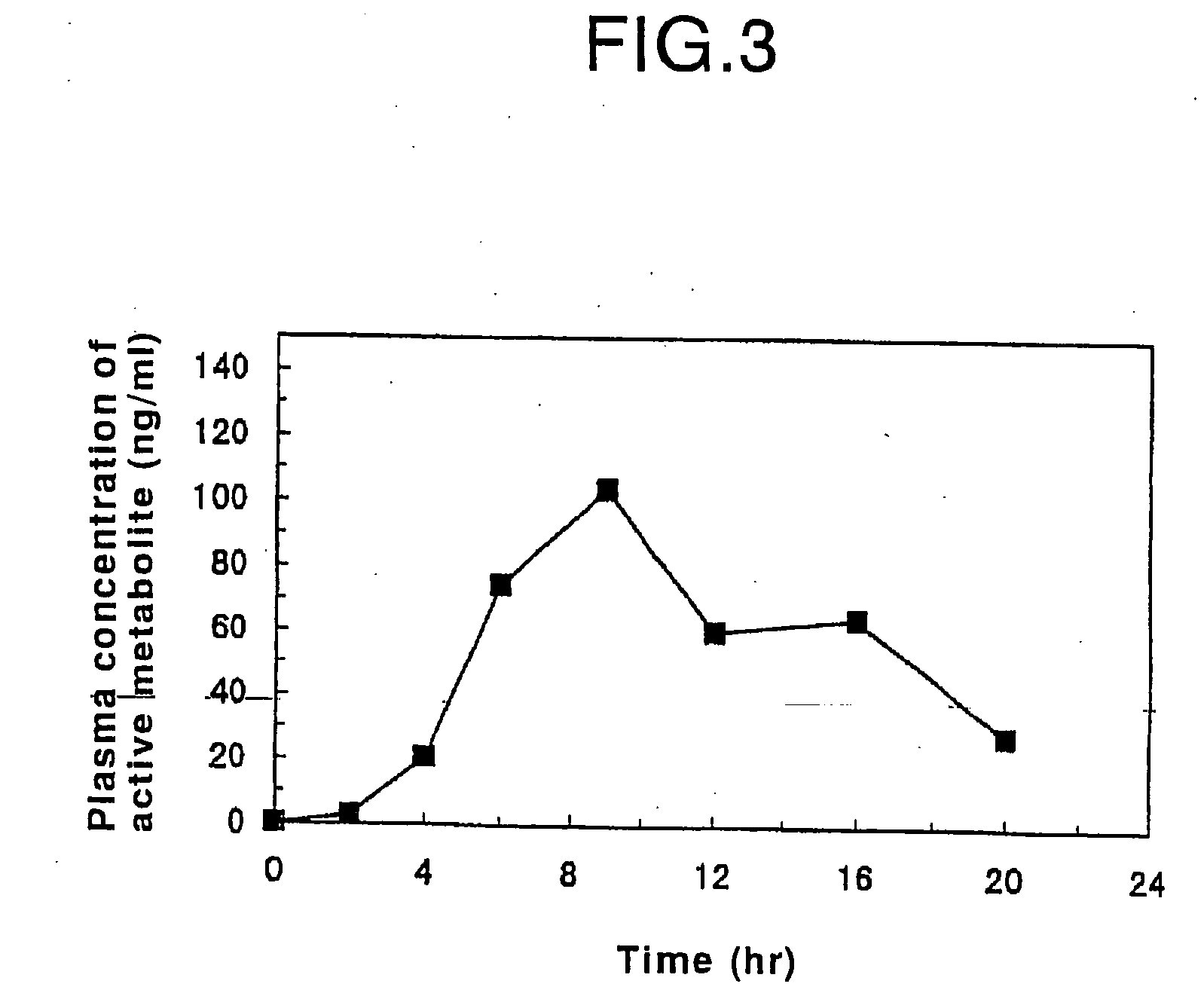
Oral sustained-release preparation of fasudil hydrochloride
a technology of fasudil hydrochloride and oral suspension, which is applied in the direction of microcapsules, cardiovascular disorders, drug compositions, etc., can solve the problems of patient forgetting to take the preparation, low compliance, and low compliance with the administration of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0194] 400 g of fasudil hydrochloride anhydride was dissolved in 600 ml of purified water to obtain an aqueous solution of fasudil hydrochloride. Using the obtained aqueous solution of fasudil hydrochloride, 350 g of NONPAREIL 105 was subjected to coating with fasudil hydrochloride by the Wurster method in the following manner. That is, 350 g of NONPAREIL 105 was charged into a LAB-1 model fluid-bed granulator (manufactured and sold by POWREX CORPORATION, Japan), which was equipped with a sprayer. The aqueous solution of fasudil hydrochloride was charged in the sprayer of the granulator. The NONPAREIL 105 was subjected to fluidization in the granulator under conditions wherein the air temperature was 80° C. and the air flow rate was 50 m3 / hr, while spraying the aqueous solution of fasudil hydrochloride into the fluid-bed of NONPAREIL 105 under conditions wherein the spraying rate was 2 ml / min and the air pressure for spraying was 2 kg / cm2 so as to coat the NONPAREIL 105 with fasudil...
reference example 2
[0195] The preparation of core member particles was conducted in substantially the same manner as in Reference Example 1 except that 400 g of fasudil hydrochloride hemihydrate was used instead of 400 g of fasudil hydrochloride anhydride and 350 g of CELPHERE CP-507 was used instead of 350 g of NONPAREIL 105, thereby obtaining 700 g of core member particles each having a particle diameter of from 600 μm to 850 μm. The same procedure as mentioned above was conducted thrice in total, so that 3 lots of the core members having a total weight of approximately 2,000 g were obtained.
reference example 3
[0196] 1,400 g of fasudil hydrochloride hemihydrate and 70 g of HPC-L were dissolved in 2,100 ml of purified water to obtain an aqueous solution of fasudil hydrochloride. Using the obtained aqueous solution of fasudil hydrochloride, 700 g of CELPHERE CP-507 was subjected to coating with fasudil hydrochloride in the following manner. That is, 700 g of CELPHERE CP-507 was charged into an MP-01 model rolling fluid-bed granulator (manufactured and sold by POWREX CORPORATION, Japan), which was equipped with a sprayer. The aqueous solution of fasudil hydrochloride was charged in the sprayer of the granulator. The CELPHERE CP-507 was subjected to fluidization in the granulator under conditions wherein the air temperature was 85° C., the air flow rate was 70 m3 / hr and the revolution rate of the rotor was 400 rpm, while spraying the aqueous solution of fasudil hydrochloride into the fluid-bed of CELPHERE CP-507 under conditions wherein the spraying rate was 8 g / min and the air pressure for s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com