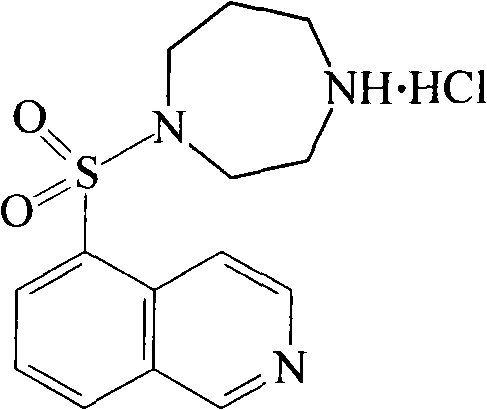
Fasudil hydrochloride compound and novel method thereof
A kind of technology of fasudil hydrochloride and compound, applied in the field of fasudil hydrochloride compound and new method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
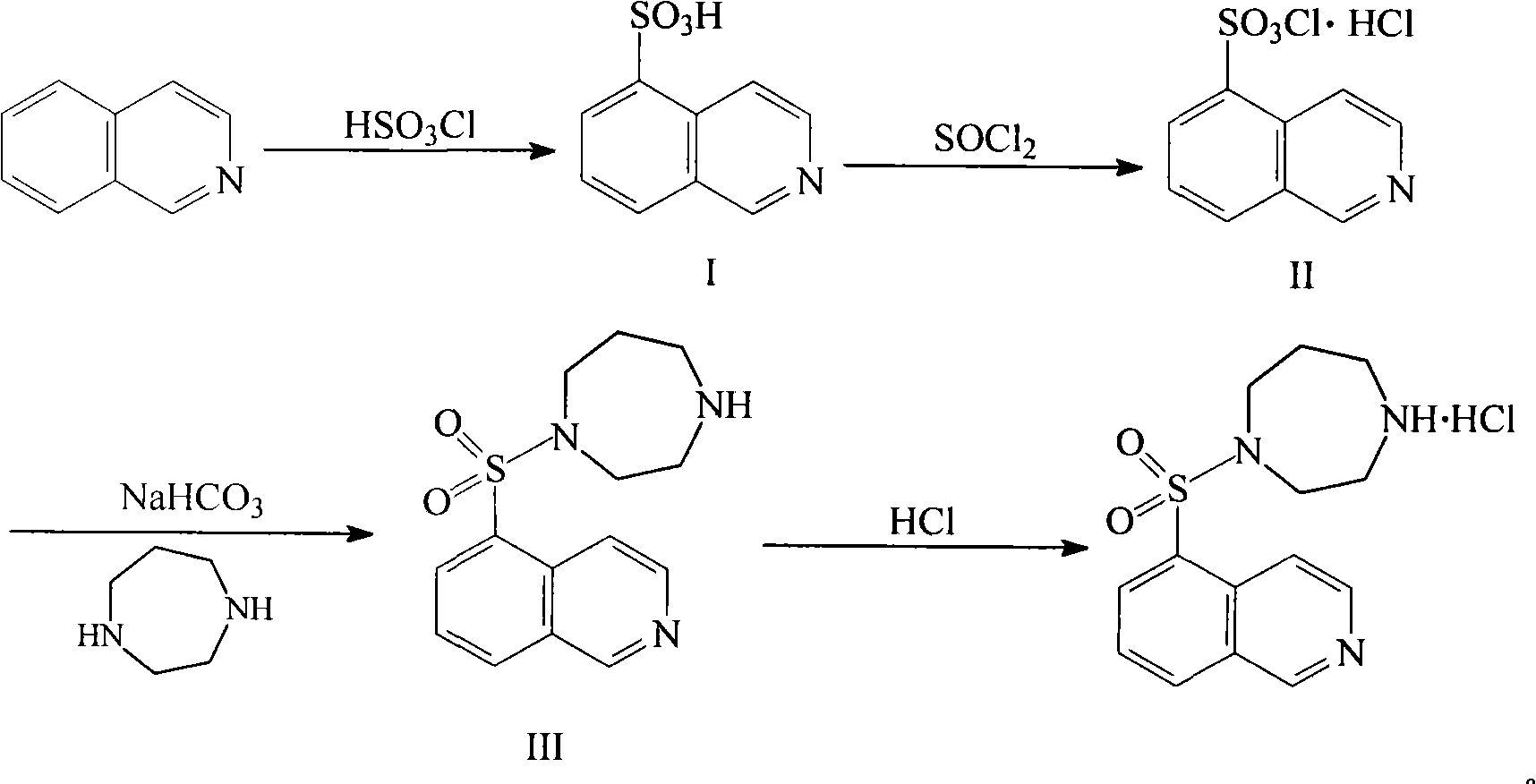
[0026] The synthesis of embodiment 1 5-isoquinolinesulfonic acid
[0027] The isoquinoline of 258g (2mol) is joined in the reaction bottle of 5L, then add the carbon tetrachloride solvent of 1L and stir to dissolve, cool to below 10 ℃ with ice water, then slowly add 256g (2.2mol) chlorosulfonic acid, Control the temperature of the reaction system below 25°C, stir and react for 8-10 hours, distill off the carbon tetrachloride solvent under reduced pressure, pour the residue into 2L of ice-water mixture, stir at below 10°C for 2-3 hours, and precipitate a solid , filtered, washed with a small amount of water first, and then washed with a small amount of methanol, and dried under vacuum at 40-50° C. to obtain 372 g of off-white solid, with a yield of 89%.
Embodiment 2
[0028] The synthesis of embodiment 2 5-isoquinolinesulfonyl chloride hydrochloride
[0029] Add 209g of 5-isoquinolinesulfonic acid, 1700ml of thionyl chloride and 1ml of dimethylformamide into a 3L reaction flask, heat to 80°C-85°C for 3-5 hours, and distill off the remaining di Chlorothionyl, then add 500ml of dichloromethane, stir, precipitate solid, filter, wash with 150ml of dichloromethane, and vacuum dry at 40-50°C to obtain 248g of solid, yield 94%.
Embodiment 3
[0030] The synthesis of embodiment 3 Fasudil hydrochloride
[0031] In a 5L reaction flask, dissolve 132g (0.5mol) of 5-isoquinolinesulfonyl chloride hydrochloride in 1200ml of ice water, then adjust the pH to 6 with saturated sodium bicarbonate solution, and then use 2.5L of dichloromethane Extract, then add 1.2L of dichloromethane solution containing 200g homopiperazine dropwise to the dichloromethane extraction solution, react at 15°C-20°C for 2-3 hours, wash twice with 500ml of purified water, no Dry over sodium sulfate, distill the solvent off under reduced pressure to obtain an oily substance, then add 500ml of methanol to dissolve it, adjust the pH to 6 with 1-2M hydrochloric acid solution, stir for 1-2 hours, concentrate to dryness under reduced pressure, then add methanol and ether (2:1) mixed solvent recrystallization, obtain 123g of Fasudil hydrochloride, yield 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com