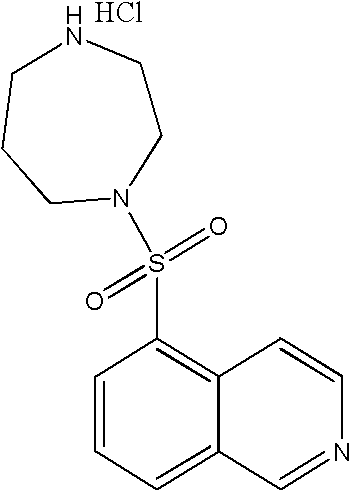
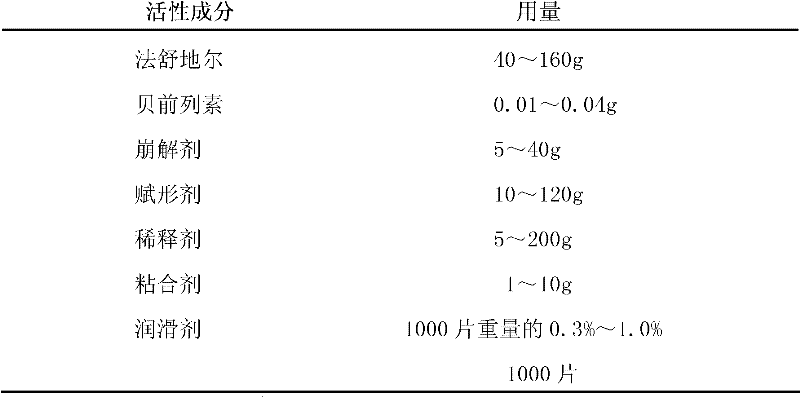
Compound preparation of Fasudil and Beraprost as well as preparation method and application thereof
A technology of beraprost and fasudil, applied in the field of pharmacy, can solve problems such as unsatisfactory effects and complicated treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074]
[0075]
[0076] Preparation:
[0077] After crushing the prescribed amount of Fasudil through a 120 mesh sieve, mix it with low-substituted hydroxypropyl cellulose, lactose, microcrystalline cellulose, starch, and calcium hydrogen phosphate that have passed through an 80 mesh sieve to obtain a mixed powder. Dissolve with appropriate amount of water, add mixed powder and mix evenly, then add 10% starch slurry to make soft material, granulate through 24 mesh sieve, ventilate and dry at 55°C, granulate with 20 mesh sieve, mix evenly with magnesium stearate, press tablet, get compound tablet core.
Embodiment 2
[0079]
[0080] Preparation:
[0081] Grind the prescribed amount of Fasudil through a 100-mesh sieve, mix evenly with low-substituted hydroxypropyl cellulose, lactose, and microcrystalline cellulose that have passed through an 80-mesh sieve, and add 2% hydroxypropyl methylcellulose as a soft material , granulated through a 24-mesh sieve, ventilated and dried at 55°C, granulated through a 20-mesh sieve and mixed evenly with magnesium stearate to obtain Granule 1 for later use.
[0082] After dissolving the prescribed amount of beraprost with an appropriate amount of water, mix it evenly with low-substituted hydroxypropyl cellulose, lactose starch, and microcrystalline cellulose that have passed through an 80-mesh sieve, and add 2% hydroxypropyl methylcellulose as a soft material , granulated through a 24-mesh sieve, ventilated and dried at 55°C, granulated through a 20-mesh sieve and mixed evenly with magnesium stearate to obtain granule 2 for later use.
[0083] The granu...
Embodiment 4
[0090]
[0091]
[0092] Dissolve the prescribed amount of fasudil and beraprost in 300ml of water to obtain solution 1; pass the prescribed amount of microcrystalline cellulose, lactose, and starch through a 100-mesh sieve and mix them evenly, place them in a vulcanized bed, and heat them with 60°C hot air Boil the mixed powder, then spray the atomized liquid of Solution 1 into the boiling mixed powder to make granules of suitable size, pass through a 40-mesh sieve for granulation, mix with magnesium stearate, fill 3# capsules, Get compound capsules.
[0093] Examples 3 and 4 prepared capsule samples, 300 capsules were filled by hand, 20 capsules were randomly weighed, and the RSD was calculated, which showed that the difference in weight between capsules was small. Dissolution is ideal. See Table 2 for the data.
[0094] Table 2
[0095]
[0096] Preparation:
[0097] Grind the prescribed amount of fasudil and beraprost through a 100-mesh sieve, low-substituted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com